Neonatal Thrombocytopenia
Review Article
Abstract :
Neonatal Thrombocytopenia is a common Problem in NICU. Platelets are produced by megakaryopoiesis and Thrombopoiesis is bone marrow in adults but in neonates Liver and Placenta are the other probable sites. Platelets appear in foetal circulation at 5 weeks of gestation and reach the adult count by 2nd trimester of Pregnancy. Tpo and Interleukin-ll are important stimulator for megakaryocytes. Normally thrombocytopenia occurs in 1-5% of Newborns, with severe thrombocytopenia in 0.1-0.5%. Neonatal Thrombocytopenia is the result of impaired Platelet production, increased Platelet destruction and sequestration or the combination of both. Increase platelet destruction can be immune mediated or non immune, associated with other diseases. Immune mediated thrombocytopenia can be neonatal Alloimmune thrombocytopenia (NAIT), Auto immune thrombocytopenia or due to maternal antiplatelet antibodies. The early onset Neonatal thrombocytopenia occurs within 72 hours and late onset after 72 hours with high risk of IVH. Thrombocytopenia is a risk factor for ICH (Intracranial Haemorrhage). IVH is more common in VLBW (Very low birth weight) infants and most are due to immune mediated Thrombocytopenia. History and clinical features are mostly over shadowed by the disease causing Thrombocytopenia. Platelet count is the mainstay of diagnosis. In Alloimmune Thrombocytopenia platelet antigen typing of father, mother and the baby is necessary. With a count below 30000/µlt. HPA (Human Platelet Antigen) compatible platelets is to be administered. In unstable eonates and with active bleeding it can be transfused below 50000/µlt. With a count of below 30000/µlt IVIg may be given regardless of bleeding.
Key Words : Neonatal Thrombocytopenia, NICU, Megakaryopoiesis, Thrombopoiesis, Tpo (thrombopoetin), Interleukin-ll, NAIT (Neonatal Alloimmune Thrombocytopenia), ICH (Intracranial Haemorrhage), IVH (Intra Venticular Haemorrhage), HPA (Human Platelet Antigen), IVIg (Intra Venous Immunoglobulin).
Introduction :
Neonatal thrombocytopenia is one of the common haematological problems encountered in neonatal intensive care unit. Though highly prevalent, little is known about the mechanisms underlying thrombocytopenia and most of them resolve spontaneously. Hence it puts the treating physician into a dilemma, when and how to treat them or ignore them. Though platelet transfusion remains the main stay of treatment, but due to risks associated with transfusion and short supply, it should be given judiciously when clinically indicated.
Megakaryopoiesis :
Megakaryopoiesis includes the production of megakaryocytes from stem cells, while thrombopoiesis is the production of platelets from megakaryocytes. Platelet formation begins in the yolk sac and then shifts to the liver and finally to the marrow.1
The most primitive progenitor cell that gives rise to megakaryocytic lineage cells is the multipotent progenitor, CFU-GEMM (Colony forming unit —granulocyte / erythrocyte / monocyte / megakaryocyte).2 The most primitive progenitor cell committed exclusively to the megakaryocytic lineage is BFU-MK (Burst forming unit megakaryocyte).2 It is CD34(cluster differentiation)positive while HLA-DR (Human Leukocyte Antigen – antigen D Related)negative. Its immediate mature progeny is CFU — MK, which is CD34, CD 41, c-mpl and HLA-DR positive.14 Thus megakaryocytic progenitor cells proliferate, under the influence of thrombopoietin and growth factors with megakaryocyte colony stimulating activity, into megakaryoblasts which further differentiate into megakaryocytes.(3,4) The function of these megakaryocytic progenitor cells is to proliferate sufficiently to ensure an adequate megakaryocyte producing cellular mass to maintain platelet production in times of need.1
Thrombopoiesis :
To assemble and release platelets, megakaryo cytes, become polyploid by endomitosis and follow a maturation program, which results in the conversion of the bulk of their cytoplasm into multiple long processes called proplatelets.5 Platelets form selectively at the ends of proplatelets as they are developing their content of granules and organelles being delivered to them as a stream from the megakaryocyte cell body. This entire process ends in rapid retraction that separates the released proplatelets from the residual cell body.1 These proplatelets extend into marrow sinusoids where the ends containing the mature platelets are extruded. Tpo (Thrombopoetin) initiates the maturation program that amplifies the megakaryocytic DNA and leads to the synthesis of platelet specific proteins.3
Differences between megakaryopoiesis in the adult and the new born:
In adult the process takes place mainly in the bone marrow. The principal site of megakaryopoiesis and thrombopoiesis in the fetus and neonate is not well defined.6 Probably the site of megakaryopoiesis and thrombopoiesis is the liver and its sinusoids in the fetal and early neonatal period.7 Microcirculation in the placenta is another site where platelet production following fragmentation could occur.8 Compared to term infants megakaryocytic precursors are abundant in preterm cord blood.3 At birth, term infants have increased megakaryocytic progenitors than adult and they have a higher clonogenic potential than adults.9But due to lower ploidy and decreased protoplatelet they produce fewer platelet than adults.4Though base line level values are same, the Tpo response to platelet consumption is low in comparison to adults.10
Except from the aforementioned, difference in megakaryopoiesis and thrombopoiesis, they are largely identical in adult and neonate. All the major megakaryocytic progenitor and precursor cells have been identified in the foetus and newborn baby.7 Platelets appear in fetal circulation from 5 weeks of gestation and several studies have shown that by the 2nd trimester the adult platelet count of 150,000- 450,000/µl is reached.7 Similar to adults the principal hemaotopoietic growth factor controlling megakaryopoiesis is Tpo or c-mpl ligand. Megakaryocyte precursors and progenitor cells from term and preterm infants proliferate extensively in response to exogenous recombinant Tpo.8 Another growth factor proposed to influence platelet production at different stages of megakaryopoiesis is interleukin 11 which has been shown to stimulate megakaryocytes of both term and preterm neonates.9 In addition to this in vitro studies show that certain hematopotetic growth factors such as IL-3, SO,, GM .C5F, FPO have a megakaryocytic progenitor cell proliferative action with multi lineage effects.10
Definition :
Platelets appear in foetal circulation as early as 5 weeks of gestation and it reaches adult value of 150,000 – 450,000/µlt by second trimester which remains constant throughout life.(11-14) Therefore irrespective of gestational age the platelet count should be above 150,000/µlt and anything less than that should be considered as thrombocytopenia. Depending on platelet count thrombocytopenia can be classified into mild (platelet count 100,000 to 150,000/µlt.), moderate (platelet count 50,000 – 99,000/ µlt.), severe (platelet count < 50,000/ µlt.) and very severe (platelet count < 30,000/ µlt.).(15- 17)
Epidemiology :
One of the commonest haematological abnormality in the neonatal periods is neonatal thrombocytopenia.8Thrombocytopenia is present in 1-5% of newborns at birth. Severe thrombocytopenia occurs in 0.1-0.5 %.( 10, 11)
Thrombocytopenia develops in 22–35% of all babies admitted to NICUs and in up to 50% of those admitted to NICUs who require intensive care. About 20% of thrombocytopenic episodes are severe and are at increased risk of haemorrhage. It is more common in preterm babies. (15-17)
Mechnisms of thrombocytopenia in neonate :
Though the exact mechanism underlying many neonatal thrombocytopenia are unknown, still they can be broadly categorised into three groups,-
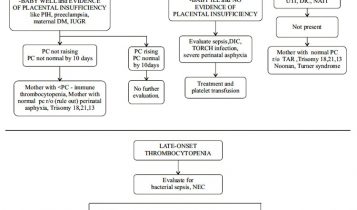
A. Impaired platelet production – In 75% of cases it is the major mechanism underlying neonatal thrombocytopenia. It may present at birth or may develop by 72 hours of life. (15-17) Most of them are preterms or babies with intra uterine growth restriction. (17, 18) The pregnancies may be complicated by placental insufficiency or fetal hypoxia. The neonates have impaired megakaryopoiesis The megakaryocytes and progenitor cells are considerably reduced at birth and thrombo-poietin (Tpo) levels are elevated. (14, 16, 18) This form of thrombocytopenia is usually mild to moderate and self limiting and resolves within 10 days in majority of cases. (18-21) The affected neonates also have a number of additional associated haematological abnormalities which help to confirm the diagnosis, including transient neutropenia, increased numbers of circulating nucleated red cells with or without associated polycythaemia, increased erythropoietin levels and evidence of hyposplenism (spherocytes, target cells and Howell-Jolly bodies). (21-23)Other rare conditions include congenital amegakayocytic thrombocytopenia, thrombocytopenia with absent radii syndrome (TAR), Fanconi’ s anemia (FA), Infants with trisomies of chromosome 13, 18 or 21 and Turners syndrome, congenital leukemia, congenital neuroblastoma and thrombocytopenia with radioulnar synostosis etc. (18,25)
B. Increased platelet destruction and sequestration – It accounts for 25 -35 % of episodes of neonatal thrombocytopenia. Mostly these occour due to transplacental passage of of maternal platelet auto and allo antibodies. Disseminated intravascular coagulation is responsible for another 10-15% of cases with very ill babies in association of severe birth asphyxia and infection.
1. Immune mediated thrombocytopenia :
Neonatal Alloimmune Thrombocytopenia (NAIT) – It occurs due to transplacental passage of maternal alloantibody against foetal platelets with inherited paternal antigen. The mother remains asymptomatic and the neonates are well appearing with features depending on severity of thrombocytopenia. In contrast to Rh(D) alloimmunization, first-born infants can be also affected. The incidence of NAIT has been estimated at 1 in 2,000 to 3,000 live births. Common manifestations include petechiae and bruising in an otherwise well-appearing infant. Intracranial bleeding occurs in 10% to 15% of NAIT.23, 24
Treatment includes platelet concentrate donated by mother and high dose intravenous immunoglobulin. All babies with NAIT should undergo cranial sonogram before discharge.
Immune thrombocytopenia due to maternal antiplatelet antibodies – In maternal Immuno thrombocytopenic purpura and systemic lupus erythematosus, passive transplacental transfer of maternal antiplatelet antibodies to the fetus and neonate occurs resulting in thrombocytopenia. The diagnosis is established by maternal history and platelet count.28 The neonate remains healthy and the platelet count correlates with that of mother. Spontaneous resolution occur over a few months depending on the clearance of maternal antibodies.
Treatment includes IVIG and platelet transfusion in severe thrombocytopenia with haemorrhage. Only transfusions are ineffective due to antiplatelet antibodies. In persistent cases a short course of prednisolone may be given.29
Autoimmune Thrombocytopenia :
It is a rare condition where neonates generate autoantibodies against its own platelet and it is usually associated with other immune disorder.29
2. Nonimmune thrombocytopenia :
It occurs mainly due to accelerated platelet destruction. It accounts for 10-15% of neonatal thrombocytopenia. The conditions are bacterial or viral sepsis, infection with HIV, Herpes simplex, cytomegalovirus, rubella etc, protozoal infections (toxoplasma, congenital malaria), Disseminated intravascular coagulation (DIC), necrotising enterocolitis (NEC), hemangiomas (Kassabach merritt syndrome), indwelling umbilical catheter, birth asphyxia and meconium aspiration syndrome in term neonates and respiratory distress syndrome in preterms, polycythemia and PPHN (persistent pulmonary hypertension).30
C. Combined mechanism :
Most of the neonates develop thrombocytopenia due to a number of mechanisms e.g. premature baby developing NEC. There is limited evidence of splenic sequestration of platelets occur in this condition.. A preterm neonate from a mother with pre-eclampsia who develops early bacterial sepsis and a baby with intrauterine growth restriction who develops NEC may both become thrombocytopenic as a result of underlying impaired platelet production (after pre-eclampsia or intrauterine growth restriction) combined with platelet consumption (during sepsis or NEC). 26
Depending on onset neonatal thrombocytopenia can be divided into two categories.
Early onset thrombocytopenia – When thrombocytopenia occurs within 72 hours after birth. It is mostly associated with maternal hypertension (PIH, preeclampsia, eclampsia), maternal diabetes, placental insufficiency, congenital infection, perinatal hypoxia and immune mediated platelet destruction. Severe thrombocytopenia is less common except in severe infection and perinatal asphyxia and DIC. (28-31)
These neonates have a predictable chronological pattern of thrombocytopenia where rarely the platelet count falls below 100,000/µl reaching its nadir by 4-5 days and recovering to more than 150,000/ µl by the end of the1st week.
In preterm and IUGR babies the early thrombocytopenia is self limiting and resolves spontaneously in 10 days.(18-21)
Late onset thrombocytopenia – When thrombocytopenia occurs after 72 hours of life. Most frequently it is caused by sepsis and necrotising enterocolitis. It may also be caused by congenital infection (TORCH), medications, and metabolic disorders. The thrombocytopenia is severe in late onset thrombocytopenia. The risk of IVH is more and may need repeated platelet transfusion. It may take several weeks to recover.23
Thrombocytopenia and Intracranial haemor- -rhage (ICH) :
It has been stressed that thrombocytopenia is one of the independent risk factors of ICH, neonatal mortality and poor neurodevelopmental outcome.35,36 The incidence of IVH is more common in VLBW infants and most of them are due to immune mediated thrombocytopenia. But it is still not demonstrated that there is decreased incidence of IVH or improved outcome with platelet transfusion if the platelet count is above 50,000/µlt. (32-34)
Approach to thrombocytopenia in newborn :
Though there is no definite approach, it can be simplified by getting the following steps.
Step -1 : Is the newborn term or preterm?
Step -2 : Is the newborn healthy looking or ill looking?
Step -3 : Is it associated with any medical condition?
Step -4 : Is there any features of congenital infection?
Step -5 : Is there any associated congenital anomaly or dysmorphic feature?
History :
Maternal history of immune thrombocytopenia, systemic lupus erythematosus, drug history, history of previously affected baby, still birth, PIH, eclampsia, intrauterine growth retardation, congeniatal and perinatal infection and history of perinatal asphyxia increases the incidence of thrombocytopenia in the neonates.
Clinical Features :
Dysmorphic features and abnormal physical findings indicating syndromic babies, jaundice, hepatosplenomegaly and retinal findings may suggest congenital intrauterine infection. Well appearing babies may have immune mediated thrombocytopenia where as sick appearing babies are associated with sepsis, NEC and/ DIC.37, 38
Investigation :
The platelet count by automated analyser should be confirmed by manual count and peripheral smear in case of thrombocytopenia (in 5% – 10% of cases). In peripheral smear examination the decreased platelet production is characterised by normal or small sized platelets where as large size platelet are seen in increased platelet destruction. Mother’s platelet count is checked in immune thrombocytopenia.31 In NAIT, platelet antigen typing of father, mother and baby and mother’s serum for ant platelet antibody should be performed. In DIC and sepsis, coagulation studies along with fibrinogen, D-dimer test and blood culture are to be performed. Bone marrow examination may be done in case of pancytopenia in CBC.31Other laboratory tests are performed according to the clinical scenario but it should not delay platelet transfusion if required.
Management:
Though there is no evidence based guideline for platelet transfusion in neonatal thrombocytopenia, identification and treatment of the cause and judicious transfusion of platelets produce good dividend.
Prophylactic platelet transfusion is not required in the first week of life for a neonate of any gestational age until the platelet count falls below 30,000/µlt except in unstable extremely preterm with previous IVH where platelet transfusion can be given with platelet count threshold at 50,000/ µlt.39
In neonates with platelet counts > 50,000/ µlt , the platelet transfusion is required in active major bleeding conditions like pulmonary hemorrhage, new or extending IVH or GI bleeding.39
Ideally HPA (human platelet antigen) compatible platelets is to be transfused. In our set up platelets should be ABO matched and leukocyte depleted. Irradiation is done to prevent transfusion related graft-versus-host disease in infants with immunodeficiency diseases.
The transfusion should be started as soon as it is received from the blood bank. It should be transfused as quickly as possible between 30 min to 2 hour.
For NAIT, along with HPA compatible platelets, IVIg is to be given and the platelet count is to be maintained above 30,000/ µlt in the first week of life. All babies should undergo neurosonogram to detect IVH.
In neonatal autoimmune thrombocytopenia, the platelet count reaches nadir at 3 to 4 days and becomes normal by 7 days in most of the cases. Prophylactic platelet transfusion is done to maintain platelet count above 30,000/ µlt along with IVIg regardless of bleeding. Sometimes a second dose of IVIg may be required in cases with persisternce of maternal antibodies (up to 12 weeks).
Conclusion :
Neonatal thrombocytopenia is a frequently encountered problem in NICU. But most of the episodes are mild to moderate and resolve spontaneously. In severe thrombocytopenia and babies with active bleeding with thrombocytopenia of any degree may be treated with platelet transfusion along with definitive treatment depending on the cause. Though IL-11, is an exciting thrombopoietic factor, is not studied well for therapeutic use.
Competing Interest : None Stated.
Funding : Nil.
References :
1. Nathan DG, Orkin Stuart H , A Thomas Look.Nathan and Oski’s Hematology of infancy and childhood. 6th ed Philadelphia: Saunders; 2003.
2. Murray NA ,Roberts IA.Circulating megakaryocytes and their progenitors in early thrombocytopenia in preterm neonates. Pediatr Res1996;40(1):112-9.
3. Dame C. Developmental biology of throm bopoietin in the human fetus andnneonate. Acta Paediatr Suppl 2002;91 (438): 54-65.
4. Dame C.Thrombopoietin in thrombocyto penias of childhood.Semin Thromb Hemost 2001;27(3):215-28.
5. Uhrynowska M, Maslanka K, Zupanska B.Neonatal thrombocytopenia:incidence, serological and clinical observations.Am J Perinatol1997;14(7):415-8.
6. Israels SJ,Rand ML,Michelson AD.Neonatal platelet function .Semin Thromb Hemost 2003;29(4): 363-72.
7. Nathan DG, Orkin Stuart H , A Thomas Look.Nathan and Oski’s Hematology of infancy and childhood. 6th ed Philadelphia: Saunders; 2003.
8. Wasiluk A. Thrombocytopoesis in health term newborns. J Perinat Med 2005;33(33):252-4. 9. Saxonhouse MA,Sola MC, Pastos KM,Ignatz ME,Hutson AD,Christensen R D ,Rimsza LM.Reticulated platelet percentages in term and preterm neonates.
10. Dame C. Developmental biology of thrombopoietin in the human fetus andnneonate. Acta Paediatr Suppl 2002; 91(438):54-65.
11. Morales WJ,Stroup M.Intracranial hemorrhage in utero due to isoimmune neonatal thrombocytopenia.Obstet Gynecol 1985;65(3 Suppl):20S-21S.
12. Roberts IA,Murray NA. Thrombocytopenia in the newborn.Curr Opin Pediatr 2003;15(1): 17- 23.
13. Raizada N,Lal A.Bhatia RC,Jain BK,Chander K,Goyal a.Neonatal thrombocytopenia due to pregnancy induced hypertension.Indian J pediatr1996;63(2):226-8.
14. Sola MC Vecchio A,Rimsza LM.Evaluation and treatment of thrombocytopenia in the neonatal intensive care unit.Clin Perinatol 2000;27(3):655-79.
15. Mehta P, Rohitkumar V, Neumann L, et al. Thrombocytopenia in the high risk infant. J Pediatr 1980;97:791–4.
16. Murray NA, Roberts IAG. Circulating megakaryocytes and their progenitors in early thrombocytopenia in preterm neonates. Pediatr Res1996;40:112–19.
17. Murray NA, Howarth LJ, McCloy MP, et al. Platelet transfusion in the management of severe thrombocytopenia in neonatal intensive care unit (NICU) patients. Transfuse Med 2002;12:35–41.
18. Watts TL, Murray NA, Roberts IAG. Thrombopoietin has a primary role in the regulation of platelet production in preterm babies. Pediatr Res 1999;46:28–32.
19. Phal G, Jauniaux E, Kinnon C, et al. Normal development of human fetal hematopoiesis between eight and seventeen weeks’ gestation. Am J Obstet Gynecol 2000:183:1029-34.
20. Holmberg L, Gustavii B, Jonsson A. A prenatal study of fetal platelet count and size with application to the fetus at risk of Wiskott Aldrich syndrome. J Pediatr 1983;102: 773–81.
21. Forestier F, Daffos F, Galacteros F. Haematological values of 163 normal fetuses between 18 and 30 weeks of gestation. Pediatr Res 1986;20:342–6.
22. Forestier F, Daffos F, Catherine N, et al. Developmental hematopoiesis in normal human fetal blood. Blood 1991;77:2360–3.
23. UpToDate [homepage on the Internet]. Causes of neonatal thrombocytopenia. [updated 2015 Mar 26; cited 2015 Jul 4]. Available from: http://www. uptodate.com/contents/causes-ofneonatal- thrombocytopenia.
24. Roganovic J, Kranjcec I. Neonatal alloimmune thrombocytopenia. Int J Pediat Health Care Adv. 2(1): 4-6.
25. Sainio S,Jarvenpaa A.L,Renlund M,Riikonen S,Teramo K,Kekomaki R.Thrombocytopenia in term infants:a population-based study.Obset Gynecol 2000;95(3):441-6.
26. Sola MC, Del Vecchio A, Rimsza LM. Evaluation and treatment of thrombocyto penia in the neonatal intensive care unit. Clin Perinatol 2000;27:655–79.
27. Castle V, Coates G, Kelton JG, et al. 111Inoxine platelet survivals in thrombocytopenic infants. Blood 1987; 70:652–6.
28. Gunnink SF, Vlug R, Fijnvandraat K, van der Bom JG, Stanworth SJ, Lopriore E. Neonatal thrombocytopenia: etiology, management and outcome. Expert Rev Hematol. 2014;7(3): 387- 95.
29. Van der Lugt NM, van Kampen A, Walther FJ, Brand A, Lopriore E. Outcome and management in neonatal thrombocytopenia due to maternal idiopathic thrombocytopenic purpura. Vox Sang.2013;105(3):236-43.
30. Cantor AB. Hemostasis in the Newborn and Infant. In: Orkin SH, Fisher DE, Look AT, Lux SE, Ginsburg D, Nathan DG, editors. Nathan and Oski’s Hematology and Oncology of Infancy and Childhood. 8th ed. Philade
31. Gunnink SF, Vlug R, Fijnvandraat K, van der Bom JG, Stanworth SJ, Lopriore E. Neonatal thrombocytopenia: etiology, management and outcome. Expert Rev Hematol. 2014;7(3): 387- 95.
32. Andrew M, Castle V, Saigal S, et al. Clinical impact of neonatal thrombocytopenia. J Pediatr 1987;110:457–64.
33. Van De Bor M, Briet E, Van Bel F, et al. Hemostasis & periventricular-intraventricular hemorrhage of the newborn. American Journal of Diseases of Children 1986;140:1131–4.
34. Andrew M, Vegh P, Caco VC, et al. A randomized, controlled trial of platelet transfusions in thrombocytopenic premature infants. J Pediatr 1993;123:285–91.
35. Beiner ME,Simchen MJ,Sivan E,Chetrit A,Kuint J,Schiff E.Risk factors for neonatal thrombocytopenia in preterm infants.Am J Perinatol 2003;20(1):49-54.
36. Mehta P, Rohitkumar V, Neumann L, et al. Thrombocytopenia in the high risk infant. J Pediatr 1980;97:791–4.
37. Bussel JB, Sola-Visner M. Current approaches to the evaluation and management of the fetus and neonate with immune thrombocytopenia. Semin Perinatol. 2009;33(1):35-42.
38. Wong W, Glader B. Neo Reviews. 2004;5(10):e444-50.
39. Murray N, Ballard S, Casbard A et al. A multicentre prospective observational study of platelet transfusion practice in neonates with severe thrombocytopenia. Blood 2006; 108:287a.
Issue: July-September 2017 [Volume 6.3]