Immunogenicity and safety of Oka varicella-zoster virus vaccine (VaripedTM) in Indian children
Original Research
Abstract:
While live attenuated Oka Merck strain varicella vaccine has been safe and effective in reducing the disease burden worldwide, it had not been investigated in the Indian population. With the purpose of obtaining licensure in India, we evaluated the safety and immunogenicity of a single dose of varicella-zoster virus (VZV) vaccine in healthy children (1–12 years), history-negative for varicella. Immunogenicity was assessed at 6 weeks postvaccinationfor childrenseronegative before vaccination, by estimating VZV-specific antibody titer usingglycoprotein-based enzyme-linked immunosorbent assay (gpELISA) and geometric mean titer (GMT). For safety assessment, all injection-site and systemic adverse events (AEs) were recorded for all childrenuntil 6 weeks postvaccination.All 130 children enrolled (mean age, 4.8 years) received 1 dose of the vaccine. Of the immunogenicity cohort(n=108), 84.3% had VZVspecific antibody titers ≥5 gpELISA units/mL (95% CI 76.0, 90.6) 6 weeks postvaccination,with overall antibody GMT of 9.9 gpELISA units/mL (95% CI 8.7, 11.2). Of the vaccinated cohort (N=130), 43.8% children experienced ≥1 AE, most were mild in intensity; the most frequently reported AEs were pyrexia (19.2%), cough (17.7%), nasopharyngitis (12.3%), and injection-site pain (6.2%). Rasheswere reported in 3.8%and injection-site reactions in 10% children. No serious AEs were reported and no participant discontinued the study because of an AE. This study demonstrateshigh immunogenicity and good tolerability of VZV vaccine in healthy Indian children. This has implications for incorporation of VZV vaccination into a universal immunization program in India to curb varicella. Clinicaltrials.gov NCT00496
Keywords: Children, Immunogenicity, Safety, Vaccine, Varicella
Introduction:
Varicella (chickenpox) is a highly contagious disease caused by infection with the varicella-zoster virus (VZV). Overall seroprevalence of anti VZV antibodies in India is 68.22%.1 The age-related seroprevalence rate of anti VZV antibodies is 29% in the age group of 1-5 years, 51.1% in 5-10 years, 71.7% in 11-15 years, 79.8% in 16-20 years, 88.1% in 21-30 years, and 91.1% in 31-40 years,1 suggesting that a considerable proportion of adolescents are infected with VZV in India.2While varicella is considered a mild disease in children with symptoms of fever, malaise, and a generalized rash lasting for about 7 to 8 days, it carries risk of serious complications, including pneumonia, central nervous system diseases, secondary bacterial infections, hemorrhagic conditions, and cardiovascular diseases or potential death.3- 6Varicella is a particularly distressing condition in older children and adults.7
Live attenuated Oka Merck varicella vaccine has demonstrated considerable safety and effectiveness in preventing varicella worldwide.8- 11The Indian Academy of Pediatrics has recommended that the first dose of vaccine for children be given at age 12–15 months and the second dose at 4–6 years. However, IAP permits that second dose of vaccine could be given anytime 3months after the first dose.In adolescents aged > 13 years and in adults, both doses can be given 4–8 weeks apart.12In the 15 years since the introduction of VZV vaccine in the United States, a major reduction was observed in varicella incidence (from 25.8 to 1.3 per 1000 person-years) in various age categories (5–9, 10–14, and 15–19 years of age), along with a reduction in hospitalization (from 2.13 to 0.25 per 100,000) with no evidence of a shift in the burden of varicella to older age groups. 8Varicella vaccination has a significant impact on lowering the disease burden and future susceptibility to VZV. Postlicensure studies have confirmed the effectiveness and tolerability of VZV vaccine. Effectiveness ranged between 86% and 94%in preventing varicella after the first dose of VZV vaccine with 100% protection against severe varicella.11,13Another study conducted over 5 years in Europe, on varicella vaccine-associated AEs, revealed that 88% AEs were non-serious10 which was similar to long-term reports in the United States.14
In developed nations such as the United States and the United Kingdom, most of the population seroconverts for varicella antibodies by adolescence; however, in several Asian countries there is a rising trend in varicella incidence,15due to the delay in implementation of the universal VZV vaccination.6Since the vaccine is not universally administered in India, reports suggest a threat for future varicella outbreaks.16,17Nearly 25% of unvaccinated adults inIndia aresusceptible to varicella16 and only 42% of the rural adults were found to be immune to VZV by the age of 25 years.18According to the Indian Academy of Pediatrics (IAP), between December 2010 and 2013, 7.7% of infectious diseases were varicella cases, of which, 58.2% were between 5 and 18 years, 18.6% between 3 and 5 years, and 15.4% between 1 and 3 years of age.19These findings suggest that a considerable proportion of varicella disease burden exists in India across allage groups.With higher complication rates,observedin adolescents and adults20this raises a health concern due to the potential increase in the incidence of varicella thereby necessitating an implementation of varicella vaccination in India.
With the purpose of obtaining licensure in India, the safety, tolerability, and immunogenicity of a single dose of VZV vaccine was evaluated in Indian children aged 12 months to 12 years who were history-negative for varicella. This study was conducted in 2005 as per the prevailing global recommendations and IAP guidelines for a single dose of VZV vaccine.12, 21
Material and method : Study design
This open-label, single-arm study (Merck protocol number 056-00, NCT00496327) was conducted at 6 centers (2 centers each at Bangalore, Mumbai, and Pune) in India between June and October 2005. The study was performed in accordance with the principles of the Declaration of Helsinki and of Good Clinical Practice. The study protocol was approved by the Independent Ethics Committees of the respective investigational centers. Written informed consent was obtained from parents or legal guardian of all participating children before enrollment in the study.
Study population
Healthy children aged 12 months to 12 years with no history of varicella, who had not received or expected to receive any form of varicella vaccine, immunoglobulin or blood products, 5 months before or within 3 months after enrollment in the study, and were not hypersensitive to any of the components of the vaccine were eligible for the study. Children were excluded if they were recently exposed (last 4 weeks) to varicella, had any medical condition which, in the opinion of the investigator, could interfere with the study objectives; had received any other inactivated or live vaccine (14 days [inactivated] or 30 days [live]) before or expected within 42 days after administration of the study vaccination; or was a pregnant or nursing female. Participants were recommended to avoid salicylates during the 6 weeks after vaccination because salicylate use in children with varicella had been associated with Reye’s syndrome. Participants could withdraw at any time or be withdrawn from the study at the discretion of the investigator because of violation of the study protocol, or administrative or safety reasons.
Study procedure and vaccine
Eligible participants received a subcutaneous 0.5 mL dose of VaripedTM (VZV vaccine live, Merck & Co., Inc., Oka/Merck strain, globally registered as VARIVAXTM), preferably into the outer aspect of the upper right arm (deltoid region) or anterolateral thigh, on Day 1.
Assessments
Immunogenicity was assessed as the percentage of individuals with VZV-specific antibody titer ≥ 5 gp ELISA units/mL at 6 weeks postvaccination. Antibody titers ≥ 5 gpELISA/ml are considered to be correlates of protection.22 A 5 mL blood sample was taken before (Day 1) and after (Day 42 [+7days]) vaccination. VZV antibody titer was determined by Merck Research Laboratories (West Point, Pennsylvania, U.S.A) using the gp ELISA assay. The GMT at 6 weeks postvaccination was calculated by taking the log of the titers, averaging over all individual values, and then backtransforming to the original scale. Only participants with a titer of < 1.25 gpELISA units/mL (seronegative status) at baseline and a measurement at 6 weeks postvaccination were included in the immunogenicity population.
All injection-site and systemic AEs were recorded from the time of enrollment to the end of follow-up. Parents/guardians were trained to record temperature from time of vaccination through day 21 post vaccination and to notify the study physician in case of high fever, varicella-like-rash, or any other unexpected reactions during the 42 days after vaccination.
The safety population included all participants enrolled in the study. All clinical charts containing information about adverse events were approved by a qualified physician. Laboratory samples were analyzed by Merck Research Laboratories.
Statistical analysis
A planned sample size of 100 evaluable participants implied that if no serious vaccinerelated adverse experiences were observed in this trial then it could be concluded that the rate of serious vaccine-related AEs in this population is no greater than 4.6%.All statistical analyses/ summaries were performed using Statistical Analysis Systems, Cary, NC, USA. For the percentage of individuals with VZV-specific antibody titer ≥ 5 gpELISA units/mL at 6 weeks postvaccination, 95% CI was calculated according to the method of Crow.23 The 95% CI for the GMT was calculated using a normal approximation. All reported AEs and baseline characteristics were summarized using descriptive statistics.
Results : Study population
Of 130 children enrolled in the study (30 children each at 2 centers in Bangalore, 20 children each at 2 centers in Mumbai and 1 center in Pune, and 10 children at 1 center in Pune), all received 1 dose of the vaccine and were included in the safety population; 4 children were withdrawn from the study and excluded from the efficacy population because of deviation from the protocol: 3 received oral poliovirus vaccine and 1 received a blood transfusion.A total of 126 (97%) children completed the study and were followed up at 6 weeks postvaccination (Figure 1). Of these, 17 children were excluded because of≥1.25 glycoprotein-based enzyme-linked immunosorbent assay (gpELISA) units/mL VZV-specific antibody titer (i.e. potentially seroprotected against varicella) before receiving varicella vaccine. One child was excluded because of missing value of the postvaccination titer. Immunogenicity population thusincluded a total of 108 participants.
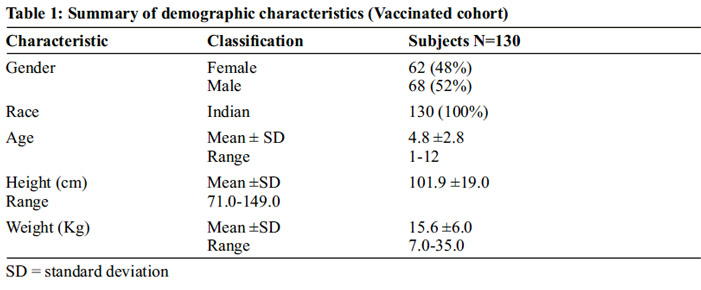
All children enrolled in the study were of Indian origin and had an even gender distribution (52% males) (Table 1). During the 6-week post vaccination follow-up period, 53 (40.8%) children received medicationsparacetamol, chlorphenamine, and amoxicillin were the most commonly used medications to treat vaccination related AEs or concurrent illnesses no concomitant vaccinations were administered.
Immunogenicity
Of the children who were originally seronegative (108), all (100%) of them in the immunogenicity population seroconverted with VZV IgG antibody titer>1.25 gpELISA units/ml at 6 weeks post vaccination. Ninety-one of them (84.3%) were found tohave VZV-specific antibody titersof≥5 gpELISA units/mL (95% confidence interval [CI] 76.0, 90.6) (Figure 2). In the immunogenicity population, the overall antibody geometric mean titer (GMT)was 9.9gpELISA units/mL (95% CI 8.7, 11.2).The distribution of VZV-specific antibody titer post vaccinationshows that more than half the children(53.7%) had VZVspecific antibody titersof≥10 gpELISA units/mL (Figure 2).
Safety
Of the 130 subjectsincluded in the safety set, majority of the vaccinated cohort(73[56.2%])did not experience an AE; 57 (43.8%) subjects reported at least one adverse event (AE)(Table 2). The most frequently reported AEs were pyrexia in 25 (19.2%) children, followed by cough (23 [17.7%]), nasopharyngitis (16 (12.3%]), and injection-site pain (8 [6.2%]). No serious AEs were reported, and no participant discontinued the study because of an AE.Total 16 (12.3%) children had AEs that were related to vaccination. Injection-site reactions such as pain, rash, or swelling were observed in 13 (10%) children.While all injection-site reactions were deemed probably or definitely related to vaccination, the intensity of these reactions were mostly mild (10 [7.7%]), with just 2 (1.5%) and 1(0.8%) participant/s reporting a moderate and severe reaction, respectively. Among skin and subcutaneous conditions,rashes were reported in 5 (3.8%)(these rashes were nonspecific and not like either VZV or wild type),lichen spinulosus in 2 (1.5%), and dermatitis atopic and pigmentation disorder in 1 (0.8%)children. In addition, upper respiratory tract infection was found only in 6 (4.6%) children.The experience of injection-site reactions in most patients (10 out of 13) occurred during the first 5 days following vaccination.
Discussion and conclusions
In the present study, a single doseVZV vaccination regimen was safe and highly immunogenic, eliciting protective seroconversion with aVZV-specific antibody titer of ≥5 gpELISA units/mL in 84.3% of Indian children who were originally history-negative for varicella. The VZVspecific antibody titer of ≥ 5 gp ELISA units/mL at 6 weeks postvaccination can render children 3.5- times less susceptible to breakthrough varicella,24 indicating that the VZV vaccination not only induced preventive mechanisms but also provided protection and resilience against breakthrough infection. The results of our study are consistent with a previous report wherein 86% children had gp ELISA levels of ≥5 units/mL at 6 weeks postvaccination.13Results of other studies conducted in the United States have shown response rates ranging between approximately 79% and 96%, with similar antibody level criteria as used in the present study.13, 24-26While there is a dearth of studies assessing the VZV vaccine in India, a multicenter study evaluating the Oka-strain varicella vaccine (VR 795) in Indian children was recently published.27 The authors chose not to use a commercial ELISA kit, stating reliability issues. They also did not perform a fluorescent antibody to membrane antigen assay, as it was not available commercially. The study found that 98.3% achieved a post-vaccination titer of VZV IgG antibody >10mIU/mL. However a true comparison cannot be made with the current study because of different baseline antibody criteria used.
An enhanced GMT was observed at 9.9 gpELISA units/mL in our study which was similar to previous studies reporting 11–13gpELISA units/mL at 6 weeks post vaccination.13, 25, 28However, it should be noted that a non-linear relationship exists between antibody titers and long-term disease protection, without a proportional increase in efficacy at high titers. Therefore, it is more important to achieve protective antibody titers than develop high levels of VZV antibody.13These immunogenicity findings can translate into vaccine effectiveness and increased likelihood that long-term immunity against varicella will be maintained in this population.
The immunogenicity of the VZV vaccine in Indian children was comparable to previous reports on Asian populations. Seroconversion rate defined by ≥ 4 fold increase in antibody titer was 94%, postvaccination in individuals from Philippines, a majority of whom were seronegative prior to vaccination.29In Japan,a single dose of live attenuated varicella vaccine provided long-term protective immunity, with a sub-set of vaccine recipients still showing presence of humoral and cellular immunity to VZV more than 20 years following immunization30and in China, VZV vaccine conferred a high level of protection against varicella with immunity lasting for at least 6 years.31
Besides being highly immunogenic, the VZV vaccine demonstrated good tolerability with majority of the children not experiencing any AEs or serious AEsin the present study consistent with previous reports.15,29,30 Similar incidences of pyrexia, cough, and injection-site AEs have been observed in previous studies evaluating the VZV vaccine.14,32,33Injection-site reactions were common following varicella vaccination and resolved quickly as observed in previous studies.7,15,22Rashes were reported in 5 (3.8%) children only during the 6 week follow-up period, of which 3 of them were injection-site rashes. All injection site rashes resolved spontaneously in 48 hours.
One reason for the proven success of the VZV vaccine is the acceptable safety profile over a long duration, even in selected immunocompromised individuals.9,13,14Yet, evaluation of breakthrough varicella is warranted; it is noteworthy that84.3% of children had a VZV-specific antibody titerof≥5 gpELISA units/mL at 6 weeks postvaccination in the present study, a level that could be considered protected against breakthrough varicella.24,34
Additional attributes that contribute to the protective effects of the VZV vaccine include the low risk of transmission of infection and robust protection even after 14 years postvaccination. 8,9,22Despite these benefits and the favorable findings, our study has its limitations. Given that the study was first of its kind in Indian population with a purpose to obtain the required registration of the vaccine, we did not assess long-term effects of the vaccine. Nevertheless, the findings from this study are expected to provide confidence to include universal varicella vaccination in India beginning at 12 months of age.
In conclusion, the VZV vaccine had an acceptable safety profile and was highly immunogenic in healthy Indian children. Oka/Merck varicella vaccine is already available globally in many countries. This study further justifies that the vaccine will be an important tool to protect Indian children from varicella.
Conflict of interest
Merck & Co., Kenilworth, NJ, USA funded the study. S Pandey is a full-time employee of MSD India. At the time of the study, G Namjoshi was a full-time employee of MSD India. S Bafna received personal fees from sponsor, Merck & Co., and CRO Biocon, as a principle investigator during the study.
Funding
This study was funded by Merck & Co., Kenilworth, NJ, USA and medical writing services were also paid for by the sponsor.
Acknowledgement
Medical writing assistance was provided by Shruti Baijal, PhD and Elphine Telles, PhD of Cactus Communications. This assistance was funded by Merck & Co., Kenilworth, NJ, USA. We would like to thank GajananNamjoshi for his contribution towards interpreting the results.
Author contribution
P.P Maiya, P.G Samdani, and M.R Lokeshwar substantially contributed to the conception and design of the study; S.D.S Rao, P.G Samdani, M.R Lokeshwar, S Bhave, and S Bafna were involved in data acquisition; S Pandey and M.R Lokeshwar analyzed the data; S Pandey, P.G Samdani, M.R Lokeshwar, S Bhave and S Bafna, interpreted the results; S Pandey and P.G Samdani substantially contributed to drafting the study; and all authors critically reviewed the manuscript for important intellectual content.
References
1. Lokeshwar MR, Agrawal A, Subbarao SD, Chakraborty MS, Ram Prasad AV, Weil J, et al: Age related seroprevalence of antibodies to varicella in India, Indian Pediatr2000, 37: 714- 719.
2. Lee BW:Review of varicella zoster seroepidemiology in India and South-east Asia, Trop Med Int Health1998, 3: 886-890.
3. Bozzola E, Tozzi AE, Bozzola M, Krzysztofiak A, Valentini D, Grandin A, et al:Neurological complications of varicella in childhood: case series and a systematic review of the literature, Vaccine,2012,30:5785-5790.
4. Ziebold C, von Kries R, Lang R, Weigl J, Schmitt HJ: Severe complications of varicella in previously healthy children in Germany: a 1- year survey, Pediatrics 2001, 108: e79-e79.
5. Cameron JC, Allan G, Johnston F, Finn A, Heath PT, Booy R: Severe complications of chickenpox in hospitalisedchildren in the UK and Ireland, Archives of disease in childhood 2007, 92: 1062-1066.
6. Papaloukas O, Giannouli G, Papaevangelou V: Successes and challenges in varicella vaccine,TherAdv Vaccines 2014, 2:39–55.
7. Drwal-Klein LA, O’Donovan CA:Varicella in pediatric patients, Ann Pharmacother 1993,27:938–949.
8. Baxter R, Tran TN, Ray P,Lewis E, Fireman B, Black S, et al: Impact of vaccination on the epidemiology of varicella: 1995-2009, Pediatrics 2014,134:24–30.
9. Gershon AA, Gershon MD: Pathogenesis and current approaches to control of varicellazoster virus infections,ClinMicrobiol Rev 2013,26:728–743.
10. Goulleret N, Mauvisseau E, Essevaz-Roulet M, Quinlivan M, Breuer J: Safety profile of live varicella virus vaccine (Oka/Merck): fiveyear results of the European Varicella Zoster Virus Identification Program (EU VZVIP), Vaccine 2010,28:5878–5882.
11. Shapiro ED, Vazquez M, Esposito D, Holabird N, Steinberg SP, Dziura J, et al:Effectiveness of 2 doses of varicella vaccine in children, J Infect Dis 2011,203:312–315.
12. Marin M, Güris D, Chaves SS, Schmid S, Seward JF: Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP), MMWR Recomm Rep 2007,56:1–40.
13. Kuter B, Matthews H, Shinefield H, Black S , Dennehy P , Watson B ,et al: Ten year followup of healthy children who received one or two injections of varicella vaccine,Pediatr Infect Dis J 2004,23:132–7.
14. Chaves SS, Haber P, Walton K, Wise RP, Izurieta HS, Schmid DS, et al: Safety of varicella vaccine after licensure in the United States: experience from reports to the vaccine adverse event reporting system, 1995-2005,J Infect Dis 2008,197:S170–7.
15. Hadinegoro SR, Hindra IS, Han HH, Gatchalian S, Bock HL. Reactogenicity and immunogenicity of a live-attenuated refrigerator-stable varicella vaccine (OKA strain) in healthy seronegative subjects age 10 months to 12 years,Southeast Asian J Trop Med Public Health 2009,40:991–999.
16. Singru SA, Tilak VW, Gandham N, Bhawalkar JS, Jadhav SL, Pandve HT.Study of susceptibility towards varicella by screening for the presence of IgG antibodies among nursing and medical students of a tertiary care teaching hospital in Pune, India,J Glob Infect Dis 2011,3:37–41.
17. Verma R, Bairwa M, Chawla S, Prinja S, Rajput M. Should the chickenpox vaccine be included in the National Immunization Schedule in India? Hum Vaccin 2011,7:874–877.
18. Mandal BK, Mukherjee PP, Murphy C, Mukherjee R, Naik T. Adult susceptibility to varicella in the tropics is a rural phenomenon due to the lack of previous exposure,J Infect Dis 1998,178:S52–S54.
19. IAP.IAP Guidebook on Immunization. 2013–14http://www.iapindia.org/files/IAP%2 0Guidelines/IAP%20Guidebook%20on%20I mmunization%202013-14.pdfAccessed on 21June 2016.
20. Kuter BJ, Ngai A, Patterson CM, Staehle BO, Cho I, Matthews H, et al: Safety, tolerability, and immunogenicity of two regimens of Oka/Merck varicella vaccine (Varivax) in healthy adolescents and adults. Oka/Merck Varicella Vaccine Study Group, Vaccine 1995,13:967–972.
21. Varicella Vaccine. In: Shah RC, Shah NK, Bansal CP, Kukreja S. editors. IAP Guidebook on Immunisation 2005-2006. National Publication House, Indian Academy of Pediatrics, Gwalior, 2007; 24
22. American Academy of Pediatrics Committee on Infectious Diseases. Prevention of varicella: recommendations for use of varicella vaccines in children, including a recommendation for a routine 2-dose varicella immunization schedule, Pediatrics 2007, 120: 221–231.
23. Crow EL. Confidence intervals for a proportion,Biometrika1956, 43: 423–435.
24. Li S, Chan IS, Matthews H, Heyse JF, Chan CY, Kuter BJ, et al: Inverse relationship between six week postvaccination varicella antibody response to vaccine and likelihood of long term breakthrough infection,Pediatr Infect Dis J 2002,21:337–342.
25. Rothstein EP, Bernstein HH, Ngai AL, Cho I, White CJ:Dose titration study of live attenuated varicella vaccine in healthy children. Pennridge Pediatric Associates,J Infect Dis 1997,175:444–447.
26. Silber JL, Chan IS, Wang WW, Matthews H, Kuter BJ:Immunogenicity of Oka/Merck varicella vaccine in children vaccinated at 12- 14 months of age versus 15-23 months of age,Pediatr Infect Dis J 2007,26:572–576.
27. MitraM, Faridi M, Ghosh A,Shah N, Shah R, Chaterjee S, et al: Bhattacharya N, Bhat G, Choudhury H, Kadhe G, Mane A, Roy S. Safety and immunogenicity of single dose live attenuated varicella vaccine (VR 795 Oka strain) in healthy Indian children: A randomized controlled study, Hum VaccinImmunother 2015,11:443–449.
28. Ngai AL, Staehle BO, Kuter BJ, Cyanovich NM, Cho I, Matthews H, et al: Safety and immunogenicity of one vs. two injections of Oka/Merck varicella vaccine in healthy children,Pediatr Infect Dis J 1996,15:49–54.
29. Barzaga NG, Florese RH, Bock HL: Reactogenicity and immunogenicity of a varicella vaccine in healthy seronegative and seropositive subjects, Southeast Asian J Trop Med Public Health 2002,33:259–267.
30. Asano Y:Varicella vaccine: the Japanese experience,J Infect Dis 1996,174:S310–S313.
31. Fu C, Wang M, Liang J, Xu J, Wang C, Bialek S: The effectiveness of varicella vaccine in China, Pediatr InfectDis J 2010,29:690–693.
32. Ferrera G, Gajdos V, Thomas S, Tran C, Fiquet A: Safety of a refrigerator-stable varicella vaccine (VARIVAX) in healthy 12- to 15- month-old children: A randomized, doubleblind, cross-over study,Hum Vaccin 2009,5:455–460.
33. Kanra G, Ceyhan M, Ozmert E:Safety and immunogenicity of live attenuated varicella vaccine in 9-month-old children,PediatrInt 2000,42:674–677.
34. Furth SL, Arbus GS, Hogg R, Tarver J, Chan C, Fivush BA: Southwest Pediatric Nephrology Study Group. Varicella vaccination in children with nephrotic syndrome: a report of the Southwest Pediatric Nephrology Study Group,J Pediatr 2003,142:145–148.
Figure legend
Figure 1. Patient disposition
Figure 2. Distributions of Varicella-zoster virus vaccine (VZV)-specific antibody concentration 6- weeks after vaccination (Immunogenicity population)
Issue: January-March 2018 [Volume 7.1]