Update on Ventilator Associated Pneumonia in Neonates and Children
Review Article
Abstract:
Ventilator-associated pneumonia (VAP) is relatively common in mechanically-ventilated children, but there is a wide variation in reported VAP rates, depending on settings and geographical regions. Surveillance definitions in children are challenging. Although these are provided by the German nosocomial infection surveillance system and an independent Dutch group, the combination of clinical and radiologic signs leaves room for interpretation. Of note, the United States Centers for Disease Prevention and Control guidelines do not offer algorithms for neonates. Despite the fact that most experts agree on the low sensitivity and specificity of existing definitions, little has changed over the past years. However, the number of studies reporting on VAP prevention programs has increased in recent years. Single interventions, such as chlorhexidine mouth wash or stress ulcer prophylaxis, were not effective. Successful prevention programs combined multiple interventions, such as hand hygiene, glove and gown use for endotracheal tube manipulation, backrest elevation, oral care with chlorhexidine, stress ulcer prophylaxis, cuff pressure maintenance where appropriate, use of orogastric tubes, avoidance of gastric distension, and elimination of non-essential tracheal suction. These multimodal strategies have proved to be successful among neonates, infants, and children. Importantly, they are applicable in high- as well as in low- and middle-income countries. This review provides an update of VAP incidence rates and summarizes current knowledge on its epidemiology, risk factors, surveillance definitions, and prevention programs in the pediatric setting.
Keywords:Ventilator-associated pneumonia Children, Neonates Healthcare-associated infection
Introduction:
Healthcare-associated infections (HAIs) are associated with morbidity, mortality, and prolonged hospitalization, and represent a serious threat to patient safety, cost and hospital and healthcare reputation. Hospitalized children and neonate being immunologically fragile are a particularly vulnerable population[1]. The incidence of HAI in adult and pediatric intensive care units (PICUs) is high. This is due to the many invasive procedures and frequent antibiotic use, which put the patients at risk for infection and promote the emergence of multidrug-resistant organisms[2]. The use of invasive devices in NICUs, such as central vascular lines and mechanical ventilation(MV), is similar to adult intensive care and thus the burden of ventilator-associated pneumonia (VAP) and other HAIs are also similar[3].
Advances in MV have enabled the provision of respiratory support to extremely preterm infants within the limits of viability. However, baro and volutrauma derived from MV cause cytoarchitectural changes and abnormal remodeling of the lung structure contributing to the development of chronic pulmonary disease. Additional complications secondary to MV include air leaks, interstitial emphysema, subglottic stenosis, and ventilator-associated pneumonia (VAP)[4]. Conspicuously, the most effective strategy proven to minimize ventilator-associated lung injury consists of reducing the duration of MV[5]. However, in spite of these recommendations, the rates of endotracheal intubation reported by the National Nosocomial Infection Surveillance System (NNIS) from January 2002 to June 2004 were still 43% in neonates with a birth weight of less than 1,000 g and 16% in newborns with a birth weight between 1,000 and 1,499 g [6].
In this review, we have tried to describe the epidemiology of VAP, risk factors study, and discuss effective prevention measures and neonatal ICUs (NICUs).
Definitions:
A uniform definition of VAP needs to have the capacity to be relevant for clinical trials, while balancing the risks of experimental therapy and sampling procedures with potential benefits for study patients[4]. If the definition of VAP is already controversial for adults, it is even more challenging for children, in particular for ventilated neonates. The starting point of the recent United States (US) Centers for Disease Prevention and Control (CDC) definitions for adults is a ventilator-associated complications (VAC), which is further narrowed towards infectious VAC and then towards possible or probable VAP, according to additional diagnostic. However, it is not clear whether this algorithm can be applied to children in different age groups and, thus, the conventional CDC definitions of hospital-acquired pneumonia for children and neonates remain valid for the time being [7]. These definitions do not specify between “ventilated” or “non-ventilated” and the use of the term “VAP” depends on the time on ventilation (48 h or longer). The German national nosocomial infection surveillance system (Krankenhaus Infektions Surveillance System [KISS]) offers a definition for very low birth weight infants in their “Neo-KISS” module [8]. A Dutch study group established their own definition for VAP in neonates, which are more inclusive than the CDC definitions [9, 10].
Table 1 summarizes the definitions of hospital-acquired pneumonia by stratifying age groups into neonates, infants (≤1 year), and children (>1 year to≤16 years). All definitions combine clinical and radiologic signs. In addition, the CDC and the European Centre for Disease Prevention and Control (ECDC) definitions further distinguish between definite, probable, and possible healthcare-associated pneumonia, based on microbiologic findings (Table 2). Clinical and radiologic findings lack sensitivity and specificity. However, tracheal aspirate cultures have also low sensitivity (31-69%) and specificity (55-100%). A positive tracheal culture alone does not discriminate between bacterial colonization and respiratory infection. Bronchoalveolar lavage (BAL) provides better results, but the range of sensitivity (11-90%) and specificity (43-100%) is large.
Table 1
Case definitions of hospital-acquired pneumonia in children stratified by different age groups
| Neonates | Onset >72 h after birth and one of the following radiologic criteria:
-new or progressive infiltrates -consolidations -adhesions or fluid in lobar fissures/pleura And Worsening gas exchange (SaO2 ?; O2 requirement ?; Ventilation parameters ?) And Four of the following signs and symptoms: -fever (>38.0°C), hypothermia (<36.5°C), or temperature instability -new onset or increasing bradycardia (<80/min) or tachycardia (>200/min) -new onset or increasing tachypnoea (>60/min) or apnoea (>20 seconds) -new onset or increasing signs of dyspnoea (retractions, nasal flaring, grunting) -increasing production of respiratory secretions and need for suctioning -purulent tracheal secretion -isolation of a pathogen in respiratory secretions -elevated C-reactive protein (>20 mg/L) I/T-ratio >0.2 |
| Infants: 2–11 months | One of the following radiologic criteria:
-new or progressive infiltrate -consolidations -cavitations -pneumatoceles And Worsening gas exchange (SaO2 ?; O2 requirement ?; Ventilation parameters ?) And Three of the following signs and symptoms: -fever (>38.0°C), hypothermia (<36.5°C), or temperature instability -leucopenia (<4000 WBC/mm3) or leucocytosis (?15,000 WBC/mm3) with left shift (?10% band forms) -new onset of purulent sputum, or change in character of sputum, or increased respiratory secretions, or increased suctioning requirements -apnoea or dyspnoea (tachypnoea, nasal flaring, retraction of chest wall, grunting) -wheezing, rales, or rhonchi -cough -bradycardia (<100/min) or tachycardia (>170/min) |
| Children: 1–16 years | One of the following radiologic criteria:
-new or progressive and persistent infiltrate -consolidation -cavitation And Three of the following signs and symptoms: -fever (>38.4°C) or hypothermia (<36.5°C) -leukopenia (<4000 WBC/mm3) or leucocytosis (?15,000 WBC/mm3) -new onset of purulent sputum or change in character of sputum or increased respiratory secretions or increased suctioning requirements -new onset or worsening cough or dyspnoea, apnoea, or tachyponea -rales or bronchial breath sounds -worsening gas exchange (SaO2 ?; O2 requirement ?; Ventilation parameters ?) |
SaO2: Oxygen saturation; I/T-ratio: immature to total neutrophil ratio; WBC: white blood cell count; ↑: increase; ↓: decrease.
Table 2
Classification of hospital-acquired pneumonia in children based on microbiological results
| Definite VAP | A child who fulfils the case definitions for hospital-acquired pneumonia (Table 1) and has one of the following:
-same pathogen isolated from bronchial secretions/BAL and blood -pathogen or virus isolated from lung biopsy, or positive growth in culture of pleural fluid, or histopathologic examination with evidence of pneumonia manifested as abscess formation, positive culture of lung parenchyma, or fungal hyphae -Pathogen or virus isolated from BAL (bacteria ?104 CFU/ml), or ?5% of BALobtained cells contain intracellular bacteria on direct microscopic exam, or protected brush with a threshold of ?104 CFU/ml, or distal protected aspirate with a threshold of ?104 CFU/ml, or positive exams for particular microorganisms (Legionella, Aspergillus, mycobacteria, Mycoplasma, Pneumocystis jirovecii) |
| Probable VAP | A child who fulfils the case definitions for hospital-acquired pneumonia (Table 1) and has one of the following:
-pathogen isolated from BAL (bacteria <104 CFU/ml) -pathogen or virus isolated from bronchial secretions, or quantitative culture of lower respiratory tract specimen (endotracheal aspirate) with a threshold of bacteria ?106 CFU/ml |
| Possible VAP | A child who fulfils the case definitions for hospital-acquired pneumonia (Table 1) with non-quantitative lower respiratory tract specimen culture or no positive microbiology, but has been treated for hospital-acquired pneumonia |
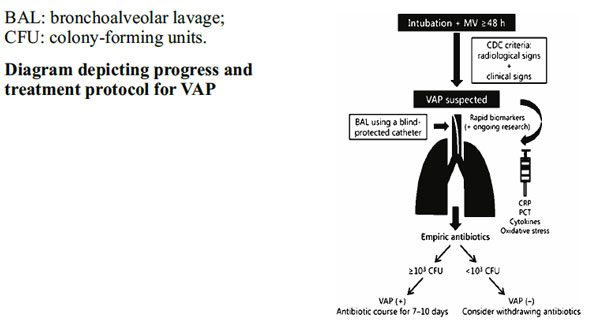
The Centers for Disease Control and Prevention (CDC; Atlanta, Ga., USA) defines VAP as ‘a nosocomial infection diagnosed in patients undergoing MV for at least 48 h’[7]. It is noteworthy that diagnosis of a VAP episode requires a combination of radiological, clinical, and laboratory criteria (table 1)[7]. However, CDC/NNIS criteria refer to infants younger than 1 year and do not define specific criteria for the newborn period in term or preterm infants. In spite of this lack of specificity, most studies of VAP performed in NICUs are based on these criteria.
Clinical Criteria
Clinical criteria for healthcare-associated pneumonia include fever, leukocytosis or leucopoenia, purulent secretions, new or worsening cough, dyspnoea, tachypnoea, crackles or bronchial breath sounds, and worsening gas exchange. These criteria are nonspecific and their sensitivity and specificity relative to the underlying pathology is poor[2]. Clinical findings must be combined with radiologic and microbiologic findings. In a study of 70 children with VAP, the modified clinical pulmonary infection score (mCPIS) of six or higher had a sensitivity of 94%, a specificity of 50%, a positive predictive value of 64%, a negative predictive value of 90%, and positive and likelihood ratios of 1.9 and 0.1, respectively [11].
Radiologic Criteria
Radiologic criteria include the presence of new or progressive pulmonary infiltrates, adhesions or fluid in lobar fissures/pleura, cavitations, air bronchograms, or pneumatoceles on chest x-rays. The presence of air bronchograms has a higher sensitivity (58-83%) than “evolving infiltrates” (50-78%) [2]. Sequential chest x-rays (days -3, 0, 2, 7) help to confirm healthcareassociated pneumonia in complex cases, such as children with underlying cardiac or pulmonary disease. It should be noted that onset and progression of pneumonia in imaging is fast, but improvement takes time.
Microbiologic Criteria
Respiratory cultures are obtained by tracheal aspirates, bronchoalveolar lavage (BAL), nonbronchoscopic BAL, or protected brush specimens (PBS) [10]. Thresholds are summarized in Table 2.
Epidemiology:
Healthcare-associated pneumonia was the most common HAI in five studies[12, 13, 14, 15,16], and second only to bacteremia in another two reports [17, 18]. The range of VAP incidence density rates in both children and neonates is large. Rates as low as 1/1000 ventilator-days and as high as 63/1000 ventilator-days have been reported (Table 3). The incidence follows a geographical distribution and depends on the type of hospital and the country income level. A surveillance study from the International Nosocomial Infection Control Consortium (INICC) identified higher VAP rates in academic compared to non-academic hospitals [19]. The same study reported higher rates in lowermiddle- income compared to upper-middle-income countries. Extreme PICU rates have been reported from India (36.2%)[20] and Egypt (31.8/1000 ventilator-days)[21]. Surveys in the USA and Germany found consistently lower rates (Table 3) [22, 23, 24]. However, high rates were reported also by high-income countries. A European multicenter study found that 23.6% of children admitted to a PICU developed VAP[25]. An Italian study identified 6.6% children with VAP among 451 on mechanical ventilation[26], and a mixed PICU in Australia identified 6.7% children with VAP among 269 on mechanical ventilation [27].
VAP is also common in the NICU and proportions between 6.8% and 57.0% of HAIs have been reported. A Spanish study identified VAP in 9.1% of 198 neonates on mechanical ventilation. In a Taiwanese NICU, 11.4% of 528 neonates had one or more HAIs, with VAP contributing to 18.6%. An INICC survey summarizing results from 30 NICUs in 15 countries reported significantly higher VAP rates in academic compared to non-academic institutions [. VAP incidence densities in an Iranian and Turkish NICU were 13.8/1000 and 11.6/1000 ventilator-days, respectively. A higher incidence was reported in another Iranian study with 42% of 38 neonates on mechanical ventilation.(28-33) Table 3 summarizes birth weight-dependent numbers from different studies.
Several studies from the USA, Italy, and Iran found that VAP prolonged mechanical ventilation by approximately 8-12 days, and this may even be as high as 56 days in extremely preterm neonates. Prolonged length of stay was the main driver of attributable costs of up to US$ 1040 in Iran and US$ 51,157 in the USA[35, 34 36]. There are no data on the attributable mortality of VAP. The mortality of HAI in the PICU is estimated to range between 5-14%, to which VAP may significantly contribute (37).
Pathogenesis and Pathogens:
It is presumed that microorganisms invading the respiratory airways and infecting the lung parenchyma may cause VAP. In his excellent review article, Garland[37] describes the possible sources of microorganisms and the pathogenic mechanisms by which they may cause VAP. Endogenous sources of microorganisms comprise colonization of the naso-/oropharynx, gastric fluid pool, or tracheal secretions. Aspiration of these contaminated fluids into the lung can result in pneumonia. On the contrary, blood-borne seeding of the lung constitutes a rare cause of VAP. Sometimes, pathogens can also reach the lung from exogenous sources such as the hands of healthcare workers, ventilator circuits, and the biofilm of endotracheal tubes (ETT).(38)
Technical difficulty in obtaining noncontaminated samples is main hinderence for the assessment of VAP etiology difficult. Apisarnt- hanarak et al.[39] reported isolation of multiple organisms from tracheal aspirates (TA) in 58% of episodes of VAP in extremely preterm neonates. In addition, Deng et al.[40] (in neonates) and Srinvasan et al.[41] (in pediatric patients) found a polymicrobial etiology in 25-40% of VAP episodes. However it should be noted that in these studies, samples were taken from ETT instead of using invasive sampling techniques of the lower respiratory tract, and therefore they might represent colonization instead of true infection. Conspicuously, when focusing on studies that used invasive techniques for sample collection, polymicrobial etiology represented only 16.7% of the VAP episodes [42].
The most common pathogens isolated in the neonatal population are Pseudomonas aeruginosa and Staphylococcus aureus[37-41]. However, isolation of other microorganisms such as Klebsiella pneumoniae and Escherichia coli has also been reported (37-41) (table 3).
There is evidence that Ureaplasma urealyticum-derived inflammation in different compartments (intrauterine, lung, blood, or brain) during a common developmental window of vulnerability contributes to preterm labor and lung and brain injury [43]. Although no mention of this agent has been made in the literature as a causative agent of VAP, it could be a confounding factor overlapping with VAP diagnosis. Therefore, Ureaplasma should be looked for in cultured samples and if present it should be taken into consideration when prescribing antibiotic therapy.
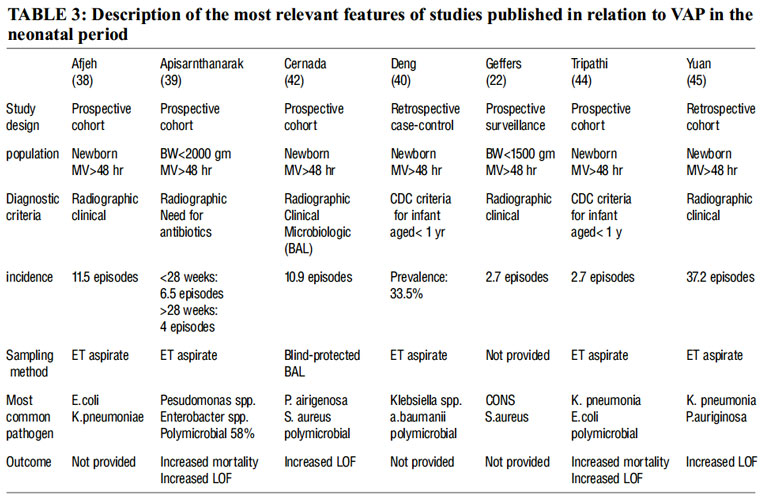
Expressed as episodes per 1000ventilator day,BW-Birth weight ,ET-endotracheal ,LOF-length of stay.
Sample Collection Methods:
The diagnostic techniques which have high false-positive rates can increase antibiotic prescription and results in selection pressure for multidrug-resistant bacteria and increased costs. Currently, both noninvasive and invasive (bronchoscopic) techniques are equally employed for sample collection. Bronchoalveolar lavage (BAL) is highly specific but invasive and only effective in experienced hands. Contrarily, noninvasive techniques such as TA are more accessible and easy to use, but they tend to overdiagnose VAP and, as a result, increase the use of antibiotics [46].
Bronchoscopic BAL and protected specimen brush (PSB) have been increasingly adopted for sample collection in VAP-suspected adults. These techniques are highly reliable as they avoid sample contamination and constitute at present the standard for microbiological sampling in the respiratory airways [48]. However in neonatology due to the small diameter of the ETT, bronchoscopic BAL and PSB are not applicable. Under these circumstances, blind-protected BAL appears to be the most reliable sampling method in the neonatal patient. Thus, in a recent prospective observational study including 198 neonates intubated for more than 48 h who fulfilled CDC criteria for VAP, lower airway secretions were collected using the BAL technique with a blindprotected catheter under sterile conditions. A total of 18 episodes of VAP were diagnosed. Among the causative agents there was a predominance of Gram-negative bacteria representing 61.9% of the total isolated bacteria, with P. aeruginosa being the most frequently isolated microorganism (19%).Other relevant pathogens were coagulasenegative staphylococci and S. aureus, while 16.7% of the cultures were polymicrobial [47].
Clinical Signs and Risk Factors:
According to CDC criteria, VAP diagnosis should only be considered after 48 h of mechanical ventilation (MV).
However, different studies have reported a wide range of days of MV before the VAP diagnosis was made. Hence, while some authors have reported VAP diagnoses in the range of 21-39 days after the initiation of MV, others have reported diagnoses as early as 4-10 days after the start of MV [47].
The most prevalent clinical sign associated with VAP refers to changes in the characteristics and volume of respiratory secretions and the appearance of purulent mucus in TA. Other signs include hypo- or hyperthermia and worsening of the respiratory distress [48].
There are a series of risk factors that predispose to VAP. Out of these, prematurity and days of MV are the most relevant ones. Prematurity is characterized by an anatomic and functional immaturity of the lung and respiratory airways together with immature antioxidant defense and immune systems. These peculiarities prompt the need for respiratory support and the tendency towards inflammation and infection, all of which favor the appearance of VAP. Very low birth weight (VLBW), described by Afjeh et al.[38] and Tripathi et al.[44] as an additional independent risk factor for developing VAP, should be considered a possible confounder since extremely-low-birth-weight infants need MV for prolonged periods of time compared to term infant.
Duration of ventilation has been acknowledged in studies performed with different sampling methods. Hence, Cernada et al.[35], Afjeh et al.[38], and Tripathi et al.[44] identified duration of MV as the most common risk factor. Of note, Cernada et al.[35] employed a novel invasive bronchoalveolar technique to avoid sample contamination for the first time in neonates.
Diagnostic Biomarkers:
Specific biomarkers of VAP allowing differentiation of pneumonia from colonization have been extensively studied in the adult population, albeit with unreliable results probably due to inconsistencies in the design of most of the studies. Therefore, studies targeting the role of biomarkers in predicting/diagnosing VAP should employ validated sampling techniques to obtain BAL fluid and secretions, disregard patients under antibiotic or corticosteroid therapy prior to sampling, apply similar sensitivity and specificity cutoff values as reported in the literature, and include a homogeneous patient population.
Procalcitonin, cytokines and chemicals related to oxidative stress have been studied extensively in adult population, but data in neonates and even in children is lacking.
Procalcitonin (PCT) is a prohormone secreted into serum as part of the systemic inflammatory response to endotoxin or mediators released in response to bacterial infections [interleukin (IL)-1?, IL-6, or TNF-?]. Interestingly, upregulation of PCT is inhibited by interferon (IFN)-?, a cytokine released in response to viral infections rendering PCT more suitable to identify bacterial infections. The PCT kinetic profile is extremely favorable for use as a clinical marker. Hence, after only 6-12 h of stimulation PCT blood levels will increase and once the infection is controlled these values will descend rapidly. PCT has been widely used for VAP diagnosis and follow-up in the adult population.
Cytokines
The presence of bacterial pathogens will be sensed by specific cytosolic receptors such as Tolllike and Nod-like receptors triggering an inflammatory response. Proinflammatory cytokines such as IL-1, IL-6, IL-8, IL-10, and TNF- ? have been evaluated in adults as markers of VAP, with discordant results. Conway Morris et al.[38] published an association between VAP and increased values of IL-1? and IL-8 measured in BAL.
Srinivasan et al.[27] evaluated the role of s- TREM and plasminogen activation inhibitor-1 (PLA-1) in the diagnosis of VAP in a PICU. They reported that elevated levels of PLA-1 had the strongest association with a clinical diagnosis of VAP and were the best biomarker to differentiate VAP from colonization.
Oxidative Stress
Following neutrophil activation through TREM-1, a burst of reactive oxygen species including hypochlorous acid (HOCl), the only source of which is the neutrophil enzyme myeloperoxidase, is generated. HOCl is too shortlived and cannot, therefore, be measured in biological materials. However, the effects of HOCl on other molecules such as tyrosine can be measured by mass spectrometry in biofluids in the form of 3-chloro-tyrosine. Another relevant candidate reflecting the prooxidant action of HOCl is glutathione sulfonamide (GSA), a stable oxidation product of reduced glutathione (GSH). GSH is extremely abundant in the cells’ cytoplasm but especially in the lung lining fluid. Therefore, GSA can be easily determined in BAL fluid. As infectious agents promote sequestration and activation of neutrophils at the inflammatory site, VAP should cause an increase in this specific biomarker in lung lavage fluid [27].
Few studies (and with disappointing results) on the use of biomarkers in neonatology have been reported.
Outcomes:
In a large European multicenter trial it was shown that nosocomial infections were associated with increased PICU/NICU and total hospital length of stay. In addition, the mortality of infected patients was also significantly increased. However, VAP patients’ mortality was no different from that of patients with infections in other organs. Other studies have confirmed that VAP is associated with increased morbidity, a longer duration of MV, and a longer hospital and/or ICU length of stay. Fischer et al.[52] reported an incidence of VAP of 9.6% in a neonatal and pediatric population after cardiac surgery and found a delay in extubation of 3.7 days attributable to VAP. Similarly, Srinivasan et al. [41],Elward(54),Gautam et al.[27] reported an increased length of stay and a longer duration of ventilation in pediatric and neonatal VAP patients with a tendency towards increased mortality that did not reach statistical significance.
Focusing on studies conducted exclusively in neonatal populations, Apistharnarak et al.[39] found VAP to be an independent predictor of mortality in VLBW infants; moreover, VAP significantly increased the NICU length of stay. Tripathi et al. [44], Yuan et al.[45], and Cernada et al.[45] reported significantly higher NICU and hospital lengths of stay in NICU patients, respectively. Although they reported higher mortality rates in VAP patients, the differences did not reach statistical significance (table 2).
Increased length of stay and morbidity causes VAP to increase hospital costs. In a 2-year study p e r f o r m e d i n P I C U p a t i e n t s , VA P w a s independently associated with increased costs, after controlling for other predictors of cost including age, underlying disease, ventilator days, and severity of illness .
Treatment:
Generally empiric antibiotics are started based on hospital data, and escalated or deescalated depending on cultures or discontinuation of antibiotics if VAP is no longer suspected. However, there are no consensus guidelines for antibiotic treatment either in neonates or in children, and empirical treatment should be selected according to the nosocomial flora and resistance patterns of each individual unit. Interestingly, in extensive drugresistant infections, aerosolized administration may be an appropriate route to deliver antibiotics and reduce systemic toxicity. Hence, Nakwan et al. [55] reported successful treatment of VAP due to Acinetobacter baumanii in a small series of preterm and term neonates with aerosolized colistin for 72 h associated with standard intravenous antibiotic therapy. No relevant side effects were noted and mortality was lower than in historical controls treated exclusively with intravenous antibiotics. Although these are promising data, more studies are needed to expand aerosolized antibiotic therapy in the newborn period, especially in drug-resistant pathogens.
The duration of antibiotic administration for VAP in the newborn period is still unknown. No published data in this regard are available in the literature. Therefore, until reliable information is accessible, the use of biomarkers of infection such as C-reactive protein or interleukins combined with the clinical course and radiological findings are the mainstay for deciding the duration of antibiotic therapy. (49)
Prevention:
Most studies of VAP in neonates focus on clinical signs, pathogens, risk factors, and outcomes. Surveillance strategies and evaluation of their effectiveness oriented toward preventing VAP in the neonatal period are scarce but of growing interest. No conclusive results have been reported on how to prevent VAP in the neonatal period; however, implementation of hygienic measures and early extubation are apparently the most efficient strategies to reduce VAP [49].
ETT and Suction:
To date, no specific recommendations related to types of ETT or airway suction have been addressed for newborn infants. However, for adults and pediatric patients the CDC and Healthcare Infection Control Practices Advisory Committee suggest the use of ETT with dorsal lumens to allow drainage of respiratory secretions, orotracheal instead of nasotracheal intubation, and a change of ventilators’ respiratory circuits only if they are visibly contaminated or do not work[57]. Interestingly, uncuffed ETT, commonly used in neonatal patients, could be a risk factor for increasing the incidence of VAP. Hence, in adults the use of polyurethane or taper-shaped cuffed ETT correlated with lower rates of VAP[57]. Intriguingly, the use of cuffed ETT in the pediatric population was associated with a reduced need for ETT changes and postextubation stridor but increased days of MV[61].
The use of ETT with nano-modified coatings apparently reduces the incidence of infections in the respiratory airways. Recently, Machado et al. [58], in a study performed in adults, reported that ETT with nano-modified coatings reduced the incidence of VAP by preventing biofilm formation and ETT colonization and providing free radical destruction of pathogens. Of note, published experiences in the neonatal period are lacking.
In relation to airway suctioning, Cordero et al.[59] compared the use of closed versus open endotracheal suction systems in ventilated neonates. No differences in the incidence of VAP or mortality between groups were found. Most nurses, however, found closed suction systems easier and faster to use and better tolerated by patients.
Hand Washing:
Routine hand washing is one of the most important strategies to reduce nosocomial infections[60]. In a 2-year-long surveillance intervention with NICU patients, increased hand hygiene compliance (from 43 to 80%) significantly reduced the incidence of respiratory infections from 3.35 to 1.06 infections per 1,000 patient days [61]. In another study, systematic use of alcoholbased gels for hand hygiene by caregivers reduced the rate of VAP in VLBW infants by 38% [62].
Rapid Extubation:
Since the duration of MV appears to be a major risk factor for the development of VAP in neonates, promptly weaning patients off the ventilator appears to be a desirable strategy to prevent VAP. Weiss M et al. [57], in a prospective study targeting reduction of the nosocomial infection rate in the NICU, implemented more aggressive strategies for the early weaning of patients off the ventilator during 3 months. When 1 year preintervention and 1 year postintervention were compared, the VAP rates decreased from 3.3/1,000 ventilator days to 1.0/1,000 ventilator days [62].
Use of Histamine 2 Receptor Antagonists or Antacids:
The use of histamine 2 receptor antagonists or antacids is believed to increase the risk of VAP as acid gastric content may make colonization with pathogenic organisms difficult. However, no differences in the incidence of VAP were found when comparing patients using or not using histamine 2 receptor antagonists or antacids [63]. There is no published experience in the neonatal period.
Selective Decontamination:
Selective decontamination consists of the establishment of a regimen of topical or intravenous antimicrobials in an attempt to reduce the burden of pathogenic bacteria in aspirated secretions. Randomized studies in pediatric patients have shown conflicting results [64]. In a prospective cohort nonrandomized study, NICU patients received oral polymixin E, tobramycin, and nystatin correctly (during the first 5 days) or incorrectly (after 5 days) or they did not receive any decolonization. Results revealed that correct selective decolonization had a protective effect toward nosocomial infections of an intestinal origin. However, a separate analysis of the impact on respiratory infections alone was not performed [65, 66]. Accordingly, no recommendation regarding selective decontamination in neonates is warranted.
Probiotics:
The loss of gut commensals such as Bifidobacterium and Lactobacilli spp. is associated with prolonged antibiotic treatments, delayed enteral feeding, or nursing in incubators and translates into proliferation of pathogenic microflora and abnormal gut colonization. Seemingly, enhancement of the enteric microbiota composition with supplementation of probiotics seems to be a good strategy to prevent sepsis and could also be applied to prevent neonatal VAP [67]. Nevertheless, a recent meta-analysis of 7 randomized controlled trials conducted in adult populations concluded that probiotics showed no beneficial effect in patients who are mechanically ventilated, did not significantly decrease the incidence of VAP, and should not be recommended for routine clinical application [67]. To date, no information regarding the use of probiotics to prevent VAP is available.
Conclusion:
VAP is common in mechanically-ventilated newborn with a wide variation of incidence and in most parts of world, be it developed or developing. Definitions are challenging in neonates since the combination of clinical and radiologic signs are difficult to interpret because of special physiology in newborn period. Gram-negative pathogens are the most common microorganisms, particularly A. baumannii and P. aeruginosa. However, there is a geographic variation with Gram-positive organisms more frequently observed in highincome compared to low- and middle-income countries. Similar to the evidence base of adult settings, a number of studies reported effective VAP prevention strategies. Multiple interventions, such as hand hygiene, glove and gown use for endotracheal tube manipulation, backrest elevation, oral care with chlorhexidine, stress ulcer prophylaxis, cuff pressure maintenance where appropriate, use of orogastric tubes, avoidance of gastric overdistension, and elimination of nonessential tracheal suction , are quite useful in prevention of VAP. These cost effective approaches can be programmed in NICU management even in resource limited set up in developing countries.
Conflict of interest: Nil
Competing interests: The authors declare that they have no competing interests.
Authors contributions:
Markanda K-reference work on definitions, Vemula N-studied epidemiology and pathogenesis, Sherdiwalla F– sample collection methods and microbiology search of references , Vasaya T– searched for preventive strategies, Kamale V– compilation and editing with final manuscript.
References:
1. Klevens RM, Edwards JR, Richards CL, Horan TC, Gaynes RP, Pollock DA, Cardo DM: Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 2007, 122: 160-166.
2. Venkatachalam V, Hendley JO, Willson DF: The diagnostic dilemma of ventilatorassociated pneumonia in critically ill children. Pediatr Crit Care Med. 2011, 12: 286-296. 10.1097/PCC.0b013e3181fe2ff.
3. Dudeck MA, Horan TC, Peterson KD, Allen- Bridson K, Morrell G, Anttila A, Pollock DA, Edwards JR: National Healthcare Safety Network report, data summary for 2011, device-associated module. Am J Infect Control. 2013, 41: 286-300. 10.1016/j.ajic. 2013.01.002.
4. Miller JD,Carlo WA: Pulmonary complications of mechanical ventilation in neonates. Clin Perinatol 2008;35:273-281, x-xi.
5. Ambalavanan N, Carlo WA: Ventilatory strategies in the prevention and management of bronchopulmonary dysplasia. Semin Perinatol 2006;30:192-199.
6. National Nosocomial Infections Surveillance System: National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control 2004;32:470-485
7. Centers for Disease Prevention and Control; National Healthcare Safety Network: CDC/NHSN Surveillance Definitions for Specific Types of Infections. 2014, http://www.cdc.gov/nhsn/PDFs/pscManual/1 7pscNosInfDef_current.pdf.
8. Krankenhaus Infektions Surveillance System: Protokoll. Surveillance nosokomialer Infektionen bei Frühgeborenen mit einem Geburts-gewicht <1.500 g (NEO-KISS). 2009,http://www.nrz-hygiene.de/fileadmin /nrz/download/NEOKISSProtokoll221209.p df.
9. van der Zwet WC, Kaiser AM, van Elburg RM, Berkhof J, Fetter WP, Parlevliet GA, Vandenbroucke-Grauls CM: Nosocomial infections in a Dutch neonatal intensive care unit: surveillance study with definitions for infection specifically adapted for neonates. J Hosp Infect. 2005, 61: 300-311. 10.1016/ j.jhin.2005.03.014.
10. Langley JM, Bradley JS: Defining pneumonia in critically ill infants and children. Pediatr Crit Care Med. 2005, 6 (Suppl): S9-S13.
11. da Silva PS, de Aguiar VE, de Carvalho WB, Machado Fonseca MC: Value of clinical pulmonary infection score in critically ill children as a surrogate for diagnosis of ventilator-associated pneumonia. J Crit Care. 2014, 29: 545-550. 10.1016/j.jcrc.2014. 01.010.
12. Abramczyk ML, Carvalho WB, Carvalho ES, Medeiros EA: Nosocomial infection in a pediatric intensive care unit in a developing country. Braz J Infect Dis. 2003, 7: 375-380.
13. Diaz-Ramos RD, Solorzano-Santos F, Padilla- Barron G, Miranda-Novales MG, Gonzalez- Robledo R, Perez JA T y: [Nosocomial infections. Experience at a third-level pediatric hospital]. Salud Publica Mex. 1999, 41 (suppl 1): S12-S17.
14. Guardia Cami MT, Jordan Garcia I, Urrea Ayala M: [Nosocomial infections in pediatric patients following cardiac surgery]. An Pediatr (Barc). 2008, 69: 34-38. 10.1157/ 13124216.
15. Lopes JM, Tonelli E, Lamounier JA, Couto BR, Siqueira AL, Komatsuzaki F, Champs AP, Starling CE: Prospective surveillance applying the national nosocomial infection surveillance methods in a Brazilian pediatric public hospital. Am J Infect Control. 2002, 30: 1-7. 10.1067/mic.2002.117039.
16. Citak A, Karabocuoglu M, Ucsel R, Ugur- Baysal S, Uzel N: Bacterial nosocomial infections in mechanically ventilated children. Turk J Pediatr. 2000, 42: 39-42.
17. Grohskopf LA, Sinkowitz-Cochran RL, Garrett DO, Sohn AH, Levine GL, Siegel JD, Stover BH, Jarvis WR: A national pointprevalence survey of pediatric intensive care unit-acquired infections in the United States. J Pediatr. 2002, 140: 432-438. 10.1067/mpd. 2002.122499.
18. Grisaru-Soen G, Paret G, Yahav D, Boyko V, Lerner-Geva L: Nosocomial infections in pediatric cardiovascular surgery patients: a 4- year survey. Pediatr Crit Care Med. 2009, 10: 202-206. 10.1097/PCC.0b013e31819a37c5.
19. Rosenthal VD, Jarvis WR, Jamulitrat S, Silva CP, Ramachandran B, Duenas L, Gurskis V, Ersoz G, Novales MG, Khader IA, Ammar K, Guzman NB, Navoa-Ng JA, Seliem ZS, Espinoza TA, Meng CY, Jayatilleke K, International Nosocomial Infection Control Consortium: Socioeconomic impact on device-associated infections in pediatric intensive care units of 16 limited-resource countries: international Nosocomial Infection Control Consortium findings. Pediatr Crit Care Med. 2012, 13: 399-406. 10.1097/PCC. 0b013e318238b260.
20. Awasthi S, Tahazzul M, Ambast A, Govil YC, Jain A: Longer duration of mechanical ventilation was found to be associated with ventilator-associated pneumonia in children aged 1 month to 12 years in India. J Clin Epidemiol. 2013, 66: 62-66. 10.1016/ j.jclinepi. 2012.06.006.
21. Rasslan O, Seliem ZS, Ghazi IA, El Sabour MA, El Kholy AA, Sadeq FM, Kalil M, Abdel- Aziz D, Sharaf HY, Saeed A, Agha H, El- Abdeen SA, El Gafarey M, El Tantawy A, Fouad L, Abel-Haleim MM, Muhamed T, Saeed H, Rosenthal VD: Device-associated infection rates in adult and pediatric intensive care units of hospitals in Egypt. International Nosocomial Infection Control Consortium (INICC) findings. J Infect Public Health. 2012, 5: 394-402. 10.1016/j.jiph.2012.07.002.
22. Geffers C, Baerwolff S, Schwab F, Gastmeier P: Incidence of healthcare-associated infections in high-risk neonates: results from the German surveillance system for very-lowbirthweight infants. J Hosp Infect. 2008, 68: 214-221. 10.1016/j.jhin.2008.01.016.
23. Leistner R, Piening B, Gastmeier P, Geffers C, Schwab F: Nosocomial infections in very low birthweight infants in Germany: current data from the National Surveillance System NEOKISS. Klin Padiatr. 2013, 225: 75-80.
24. Edwards JR, Peterson KD, Andrus ML, Dudeck MA, Pollock DA, Horan TC: National Healthcare Safety Network (NHSN) Report, data summary for 2006 through 2007, issued November 2008. Am J Infect Control. 2008, 36: 609-626. 10.1016/j.ajic.2008.08.001.
25. Raymond J, Aujard Y: Nosocomial infections in pediatric patients: a European, multicenter prospective study. European Study Group Infect Control Hosp Epidemiol. 2000, 21: 260- 263. 10.1086/501755.
26. Patria MF, Chidini G, Ughi L, Montani C, Prandi E, Galeone C, Calderini E, Esposito S:Ventilator-associated pneumonia in an Italian pediatric intensive care unit: a prospective study. World J Pediatr. 2013, 9: 365-368. 10.1007/s12519-013-0444-y.
27. Gautam A, Ganu SS, Tegg OJ, Andresen DN, Wilkins BH, Schell DN: Ventilator-associated pneumonia in a tertiary paediatric intensive care unit: a 1-year prospective observational study. Crit Care Resusc. 2012, 14: 283-289.
28. Elster T, Beata Czeszynska M, Sochaczewska D, Konefal H, Baryla-Pankiewicz E: [Analysis of risk factors for nosocomial infections in the neonatal intensive care unit of the Pomeranian Medical University in Szczecin in the years 2005-2008]. Ginekol Pol. 2009, 80: 609-614.
29. Couto RC, Pedrosa TM, Tofani Cde P, Pedroso ER: Risk factors for nosocomial infection in a neonatal intensive care unit. Infect Control Hosp Epidemiol. 2006, 27: 571-575. 10.1086/504931.
30. Helwich E, Wojkowska-Mach J, Borszewska- Kornacka M, Gadzinowski J, Gulczynska E, Kordek A, Pawlik D, Szczapa J, Domanska J, Klamka J, Heczko PB: Epidemiology of infections in very low birth weight infants. Polish Neonatology Network research. Med Wieku Rozwoj. 2013, 17: 224-231.
31. Mahfouz AA, Al-Azraqi TA, Abbag FI, Al- Gamal MN, Seef S, Bello CS: Nosocomial infections in a neonatal intensive care unit in south-western Saudi Arabia. East Mediterr Health J. 2010, 16: 40-44.
32. Broughton EI, Lopez SR, Aguilar MN, Somarriba MM, Perez M, Sanchez N: Economic analysis of a pediatric ventilatorassociated pneumonia prevention initiative in Nicaragua. Int J Pediatr. 2012, 2012: 359-430.
33. Yapicioglu H, Ozcan K, Sertdemir Y, Mutlu B, Satar M, Narli N, Tasova Y: Healthcareassociated infections in a neonatal intensive care unit in Turkey in 2008: incidence and risk factors, a prospective study. J Trop Pediatr. 2011, 57: 157-164. 10.1093/tropej/fmq060.
34. Moradi M, Nili F, Nayeri F, Amini E, T. E: Study of characteristics, risk factors and outcome for ventilator associated pneumonia in neonatal intensive care unit patients. Tehran Univ Med J. 2013, 71: 373-381.
35. Rosenthal VD, Maki DG, Jamulitrat S, Medeiros EA, Todi SK, Gomez DY, Leblebicioglu H, Abu Khader I, Miranda Novales MG, Berba R, Ramirez Wong FM, Barkat A, Pino OP, Duenas L, Mitery Z, Bijie H, Gurskis V, Kanj SS, Mapp T, Hidalgo RF, Ben Jaballah N, Raka LGikas A, Ahmed A, le TA T, Guzman Siritt ME, INICC Members: Int. Nosocomial Infection Control Consortium (INICC) report, data summary for 2003-2008, issued June 2009. Am J Infect. 2010, 38: 95- 104. 10.1016/j.ajic.2009.12.004. e2
36. Bigham MT, Amato R, Bondurrant P, Fridriksson J, Krawczeski CD, Raake J, Ryckman S, Schwartz S, Shaw J, Wells D, Brilli RJ: Ventilator-associated pneumonia in the pediatric intensive care unit: characterizing the problem and implementing a sustainable solution. J Pediatr. 2009, 154: 582- 587 e582. 10.1016/j.jpeds.2008.10.019.
37. Garland JS, Uhing MR: Strategies to prevent bacterial and fungal infection in the neonatal intensive care unit. Clin Perinatol. 2009, 36: 1- 13. 10.1016/j.clp.2008.09.005.
38. Afjeh SA, Sabzehei MK, Karimi A, Shiva F, Shamshiri AR: Surveillance of ventilatorassociated pneumonia in a neonatal intensive care unit: characteristics, risk factors, and outcome. Arch Iran Med 2012;15:567-571.
39.Apisarnthanarak A, Holzmann-Pazgal G, Hamvas A, Olsen MA, Fraser VJ: Ventilatorassociated pneumonia in extremely preterm neonates in a neonatal intensive care unit: characteristics, risk factors, and outcomes. Pediatrics 2003; 112:1283-1289.
40. Deng C, Li X, Zou Y, et al: Risk factors and pathogen profile of ventilator-associated pneumonia in a neonatal intensive care unit in China. Pediatr Int 2011;53:332-337.
41. Srinivasan R, Asselin J, Gildengorin G, Wiener-Kronish J, Flori HR: A prospective study of ventilator-associated pneumonia in children. Pediatrics. 2009, 123: 1108-1115. 10.1542/peds. 2008-1211.
42. Cernada M, Aguar M, Brugada M, Gutierrez A, Lopez JL, Castell M, Vento M: Ventilatorassociated pneumonia in newborn infants diagnosed with an invasive bronchoalveolar lavage technique: a prospective observational study. Pediatr Crit Care Med. 2013, 14: 55-61. 10.1097/PCC.0b013e318253ca31.
43. Rose M. Viscardi, Ureaplasma species: Role in Diseases of Prematurity, Clin Perinatol. 2010 Jun; 37(2): 393-409. Author manuscript; available in PMC 2011 Jun 1.Published in final edited form as:doi: 10.1016/j.clp.2009. 12.003PMCID: PMC2891804NIHMSID: NIHMS166346
44. Tripathi SH, Malik GK, Jain A, Kohli N: Study of ventilator associated pneumonia in neonatal intensive care unit: characteristics, risk factors and outcome. Internet J Med Update 2010;5:12-19.
45. Yuan TM, Chen LH, Yu HM: Risk factors and outcomes for ventilator-associated pneumonia in neonatal intensive care unit patients. J Perinat Med 2007;35:334-338.
46. Garland JS: Strategies to prevent ventilatorassociated pneumonia in neonates. Clin Perinatol 2010;37:629-643.
47. Rosenthal VD, Maki DG, Jamulitrat S, Medeiros EA, Todi SK, Gomez DY, Leblebicioglu H, Abu Khader I, Miranda Novales MG, Berba R, Ramirez Wong FM, Barkat A, Pino OP, Duenas L, Mitery Z, Bijie H, Gurskis V, Kanj SS, Mapp T, Hidalgo RF, Ben Jaballah N, Raka LGikas A, Ahmed A, le TA T, Guzman Siritt ME, INICC Members: Inte. Nosocomial Infection Control Consortium (INICC) report, data summary for 2003- 2008, issued June 2009. Am J Infect. 2010, 38: 95-104. 10.1016/j.ajic.2009.12.004. e2
48. Baltimore RS: The difficulty of diagnosing ventilator-associated pneumonia. Pediatrics 2003;112:1420-1421. External Resources Pubmed/Medline (NLM),CrossRef (,ISI Web of Science),Morris AC, Kefala K, DOI)
49. Cernada M.a, b · Brugada M.a, b · Golombek S.c · Vento M.a, b Ventilator-Associated Pneumonia in Neonatal Patients: An UpdateaNeonatal Research Unit, Health Research Institute La Fe, and bDivision of Neonatology, University and Polytechnic Hospital La Fe, Valencia, Spain;cRegional Neonatal Center, Maria Fareri Children’s Hospital, Westchester Medical Center, New York Medical College, Valhalla, N.Y., USA© 2013 S. Karger AG, Basel
50. Apisarnthanarak A, Holzmann-Pazgal G, Hamvas A, Olsen MA, Fraser VJ: Ventilatorassociated pneumonia in extremely preterm neonates in a neonatal intensive care unit: characteristics, risk factors, and outcomes. Pediatrics 2003;112:1283-1289. Geffers C, Baerwolff S, Schwab F, Gastmeier P: Incidence of healthcare-associated infections in high-risk neonates: results from the German surveillance system for very-low-birthweight infants. J Hosp Infect 2008;68:214-221.
51. Biomarkers of Neonatal Sepsis:Clarissa Deleon, Karen Shattuck, Sunil K. Jain in NeoReviews May 2015, 16 (5) e297-e308; DOI: 10.1542/neo.16-5-e297
52. Vanlaere I, Libert C: Matrix metalloproteinases as drug targets in infections caused by gram-negative bacteria and in septic shock. Clin Microbiol Rev 2009;22:224-239, table of contents.
53. Fischer JE, Allen P, Fanconi S: Delay of extubation in neonates and children after cardiac surgery: impact of ventilatorassociated pneumonia. Intensive Care Med 2000;26:942-949.
54. Elward AM, Warren DK, Fraser VJ: Ventilator-associated pneumonia in pediatric intensive care unit patients: risk factors and outcomes. Pediatrics 2002;109:758-764..
55. Nakwan N, Wannaro J, Thongmak T, et al: Safety in treatment of ventilator-associated pneumonia due to extensive drug-resistant Acinetobacter baumanniiwith aerosolized colistin in neonates: a preliminary report. Pediatr Pulmonol 2011;46:60-66.
56. Tablan OC, Anderson LJ, Besser R, Bridges C, Hajjeh R: Guidelines for preventing health care-associated pneumonia, 2003: recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee. MMWR Recomm Rep 2004;53:1-36.
57. Weiss M, Dullenkopf A, Fischer JE, Keller C, Gerber AC: Prospective randomized controlled multi-centre trial of cuffed or uncuffed endotracheal tubes in small children. Br J Anaesth 2009;103:867-873.
58. Machado MC, Cheng D, Tarquinio KM, Webster TJ: Nanotechnology: pediatric applications. Pediatr Res 2010;67:500-504.
59. Cordero L, Sananes M, Ayers LW: Comparison of a closed (Trach Care MAC) with an open endotracheal suction system in small premature infants. J Perinatol 2000;20:151-156.
60. Pittet D, Dharan S, Touveneau S, Sauvan V, Perneger TV: Bacterial contamination of the hands of hospital staff during routine patient care. Arch Intern Med 1999;159:821-826.
61. Won SP, Chou HC, Hsieh WS, et al: Handwashing program for the prevention of nosocomial infections in a neonatal intensive care unit. Infect Control Hosp Epidemiol 2004;25:742-746.
62. Rogers E, Alderdice F, McCall E, Jenkins J, Craig S: Reducing nosocomial infections in neonatal intensive care. J Matern Fetal Neonatal Med 2010;23:1039-1046.
63. Yildizdas D, Yapicioglu H, Yilmaz HL: Occurrence of ventilator-associated pneumonia in mechanically ventilated pediatric intensive care patients during stress ulcer prophylaxis with sucralfate, ranitidine, and omeprazole. J Crit Care 2002;17:240-245.
64. Ruza F, Alvarado F, Herruzo R, et al: Prevention of nosocomial infection in a pediatric intensive care unit (PICU) through the use of selective digestive decontamination. Eur J Epidemiol 1998;14:719-727.
65. Herruzo-Cabrera R, Garcia Gonzalez JI, Garcia-Magan P, del Rey-Calero J: Nosocomial infection in a neonatal intensive care unit and its prevention with selective intestinal decolonization: a multivariant evaluation of infection reduction. Eur J Epidemiol 1994;10:573-580.
66. Manzoni P, De Luca D, Stronati M, et al: Prevention of nosocomial infections in neonatal intensive care units. Am J Perinatol 2013;30:81-88.
67. Gu WJ, Wei CY, Yin RX: Lack of efficacy of probiotics in preventing ventilator-associated pneumonia probiotics for ventilatorassociated pneumonia: a systematic review and meta-analysis of randomized controlled trials. Chest 2012;142:859-868.
Issue: January-March 2017 [Volume 6.1]
