The Prospects of ORS And Miles Ahead
Review Article
Corresponding Author: Dr,Gadadhar Sarangi, Consulting paediatrician, THE CHILD, Cuttack, Odisha. Email:drgadadhars@gmail.com.
Received: 14, May, 2019; Reviewed: 30, May, 2019; Accepted: 22, June, 2019.
Citation of article: Gadadhar Sarangi , Nimain Mohanty,Nitin Kadam .The Prospects of ORS And Miles Ahead; New Indian Journal of Pediatrics, 2019;8.3.
Abstract: Comprehensive oral therapy has decreased the deaths in last 3 decades. ORS has reduced the need for intravenous fluids and consequently hospitalization. The cost of ORS is also low and is within the buying capacity of most people in developing countries. However, it cannot be used with intractable vomiting or diarrhea. It is of limited use in severe dehydration with circulatory compromise but can be given after stabilization with intravenous fluids. As “some” dehydration does not produce appreciable electrolyte imbalance there is no need of electrolyte estimation for initiation or follow up therapy with ORS solution.
However, certain insights may improve efficacy and acceptance of suitable rehydration solution in developing world. The keeping quality of the solution after reconstitution in potable water at different temperatures and humidity is not calculated. For 75 mEq/L of sodium the glucose requirement will be 37.5 mmol/L as 2 atoms of sodium constitutes a molecule. The initial experiment of Sladden and Dawson points to the same. Freshly prepared rice pulp or puffed rice pulp is a better candidate to replace glucose and is time honouredly practiced by people. Research in this direction is scanty. The existing intraluminal sodium and water is not taken into consideration. Theoretically simple glucose solution will allow absorption of fluid as sodium is already present in Jejunum. Addition of resistant starch to ORS as it does not increase the Osmolarity significantly should be brought under the ambit of research. In ReSoMal the high amount of dextrose should be scanned for its effect on the gut as potassium does not need glucose for absorption. The high Potassium also should to be tested for acceptability.
Keywords: ORS, diarrhoea, osmolarity, dehydration
Introduction: Diarrhoea still continues to be a major cause of hospitalization and death is under fives and have severe economic consequences. Diarrhoea is the second leading cause of death in children under five years old. Every year diarrhoea kills around 525000 under five children. Globally there are nearly 1.7 billion cases of childhood diarrhoea every year. It is also the major cause of Malnutrition in under fives1. From 2000 to 2016 the toll of annual number of deaths from diarrhoea in under fives had decreased by 60%. May more could have been saved through simple interventions2. It counts for 8% deaths in this age. In 2015 under five children death in India from diarrhoea accounted for 10% (117,285) of all deaths. There is a 52% reduction in mortality over the decade. The prevalence of diarrhoea remained high at 9.2%.3 The mortality is much less than the pre ORS era4. ORS introduction has reduced the mortality significantly in cholera and other diarrhoeal diseases5. It has been accepted as one of the most important medical advances of the last century6.
Definition: The Oral Rehydration Salts solution essentially involves the use of balanced electrolyte solutions with glucose to reverse dehydration and maintain hydration during diarrhoeal illness. With increase in understanding the process and effects of diarrhea, establishment of feeding and early nutrition have become an integral part of Oral hydration salts (ORS) solution theraphy7.
Historical Perspectives:
An intravenous fluid for the treatment cholera was first introduced by T. Latta in 1832. The first ever oral electrolyte solution was used by HE Harrison in 19548. In 1960 Curran described the coupled transport of Glucose and sodium providing the scientific basis for therapy with ORS solution9. Sladden and Dawson in 1969 showed the inter-relationship of glucose, sodium and water absorption in normal human jejunum and established that sodium and glucose in equimolar concentration effects maximum water absorption and showed adverse results with high or low glucose concentration10. This inspired a multitude of clinical trials in using ORS in cholera. In 1970s world Health Organisation (WHO) settled on a standardized formula for diarrhea of any aetiology, for all ages and started aggressively promoting it from 1978. In multicentric studies conducted from 1995-1998 it was pointed out that Reduced Osmolatity ORS is more acceptable in childhood non-choleric diarrhea. In 2001 WHO/UNICEF proposed to bring it to universal use replacing the standard WHO ORS shllution11. WHO and UNICEF in a joint statement advocated for addition of Zinc with ORS for the treatment of acute diarrhoea12. There is a place of oral rehydration solution to reduce acute kidney injury in burn victims is proved with experimental model13.
ORS with low sodium, high potassium and high Glucose content has been advocated to treat dehydration in severe acute malnutrition under the name of ReSoMal14.
Physiology of intestinal water and electrolyte transport: Understanding the physiologic basis of Oral Electrolyte Therapy is a necessary pre-requisite for its use. The important anatomic components of Intestinal water and Electrolyte transport are the villous with mature enterocytes at the tip and the immature crypt cells at the bottom. The brush border of the enterocyte increases the surface area. The basolateral membrane is responsible in transmitting electrolytes and organic substrates actively through Na+ – K+ – ATPase (Sodium Pump) or passively to the paracellular space. The paracellular space stationed in between two enterocytes acts as the middle compartment between lumen and the capillary of the Gut and transfers fluid and electrolytes to the vascular compartment15. The tight junction which binds the apices of two adjacent enterocytes is crucial in fluid and electrolyte absorption. It is leakier at the jejunal area and tighter in the colonocytes. Depending upon the osmotic gradient, fluid in the duodenum and upper part of jejunum may enter the lumen from the body and vice-versa may occur depending upon the osmolar situation.
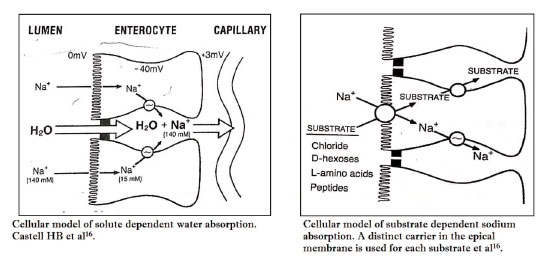
Water transport: The net influx of water to the body from the bowel is the final result of bidirectional movement of water-molecules from lumen to blood (Absorption) and from blood to Lumen (Secretion). Maximum water absorption is passive in nature and occurs as a result of Osmotic difference across the epithelial bilayer lipid membrane. Because of the hypertonicity in the paracellular space, water moves passively from the intestinal lumen through the tight junction into the space and creates increased hydrostatic pressure in the area which propels water to the vascular compartment through the paracellular pathway (Fig 1)16. Water is also dragged with sodium into the cell through the brush border and then through the basolateral membrane to the paracellular space.
Sodium Absorption: Substrate dependent sodium absorption accounts for maximum absorption of sodium and it is intact in acute diarrrhoea. The carrier mediated transport process is confined to the small intestine. Monosaccharides, amino acids and chloride bind to different carrier proteins present in the apical membrane and enter into the cell. Sodium is extruded to the paracellular space by sodium pump and monosacharides /amino acids, due to the concentration gradient diffuse to the space. Thus the cell interior becomes negatively charged with reduced sodium concentration in comparison to gut lumen which favour electrogenic transport of sodium (Fig 2)16.
Electrogenic transport accounts for 20% sodium absorption and is present in the colon also. Sodium absorption in the jejunum is enhanced due to the luminal bicarbonate which accepts a hydrogen ion in exchange with sodium that enters the epical cell.
Jejunum is responsible for a large proportion of sodium absorption due to the solvent drag through the leaky tight junction.17. It also permits the back leak of sodium with water if the intraluminal concentration of sodium is low. Though the carrier mediated sodium absorption conserves a lot of sodium at this area yet with low sodium in luminal contents there is excess secretion of sodium to the gut which may get lost in the stool exposing to the risk of hyponatremia. In the colon the tight junction is not leaky. Therefore, electrogenic sodium transport is intact in very low level of sodium content which should be more than the intracellular sodium concentration.
High Osmolar fluid in the jejunal area may drag more fluid from the body to the lumen which may overwhelm the capacity of absorption resulting in persistence of diarrhea and more fluid requirement to achieve good hydration.
Sodium is absorbed in to the enterocyte by co-transport with glucose via SGLT1 (Sodium Glucose Co transporter) Protein. 2 Sodium ions are transported with one glucose or organic molecule and attract hundreds of water molecules for Osmotic equilibrium. In the basolateral membrane 3 sodium ions are pumped out from the cells against 2 Potassium ions getting in with sodium-Potassium (Na+ -K+) Pump18. The normal sodium concentration after a meal in Jejunum is 110 mEq/L19.
Potassium Absorption:
Majority absorption occurs in small intestine by passive mechanism. It is not disturbed by diarrhea perse. Obligate Potassium loss takes place with increased unabsorbed anions. Loss of muscle mass in malnutrition also contributes to K+ loss. Vomiting causes increased renal excretion of K+20.
Pathophysilogy of acute diarrhoea and implications of therapy: Secretary diarrhea is due to increased secretion of chloride, sodium and water in the gut as a result of endotoxin stimulated opening of the chloride channels at the crypt cells. However, except coupled sodium and chloride absorption all other mechanisms of sodium absorption remain intact. The enterocyte morphology is not affected, thereby making it possible to rehydrate with ORS solution.21.
Osmotic diarrhea results in decreased absorption. There is loss of villous epical membrane due to inflammation but inspite of damage; sufficient enterocytes are preserved to carry out the function of glucose and sodium absorption. Secretary diarrhoea, the prototype being cholera is often encountered in developing countries, while osmotic diarrhea with rota virus affection being the commonest one seen in developed nations. The stool sodium loss in secretory diarrhea is higher than in osmotic diarrhea. The stool sodium and volume loss appears to diminish over time in both types22. Therefore, ideally the sodium requirement of secretory diarrhea should be more than osmotic diarrhea. The maintenance and ongoing fluid loss should have less sodium than the fluid of dehydration correction.
Compostion of ORS : The important goal of therapy in diarrhoea is to correct dehydration by replacing water loss. Correction of sodium deficit, therapy for acidosis, replacement of potassium loss and to provide chloride for ionic balance are the important considerations apart from fluid replacement. The intactness of sodium and glucose coupled transport stimulating water absorption, paved way for ORS solutions. Maximum absorption was shown to occur at a molar ratio of sodium to glucose close to one. This mechanism is intact in secretory diarrhea and sufficiently intact in Osmotic diarrhoeas.
GE. Sladden and AM Dawson had shown in human experimental model with perfusion of saline with different strength of Glucose that maximum water absorption occurred with 84 mmol/L of Glucose in saline with 154 mmol/L of sodium, using 30 cm length of human Jejunum23.
Initially WHO proposed a water and electrolyte solution close to the ionic loss in cholera stool and the effect of such therapy was demonstrated in various areas of the globe. The same solution offered to individuals of different ages with varying aetiology also produced good result. The standard ORS solution has 90 mEq/L of sodium, 20 mEq/L of Potassium, 30 mEq/L of bicarbonate or 10 mEq/L of tribasic sodium citrate, 80 mEq/L of chloride and 111 mmol/L of glucose. The citrate ORS has an Osmolarity of 311 mOsm/L24. Bicarbonate was replaced by citrate to reduce interaction with glucose to form edols and enols.
This single formulation was recommended by WHO and UNICEF for more than 30 years to treat and prevent dehydration from diarrhea of any aetiology including cholera and in individuals of any age25. Used according to recommended guidelines with ready access to plain water or breast feeding during oral rehydration it can be effective in both developing as well as developed world.
It has been well established that ORS solution does not reduce stool output, duration, frequency or severity of diarrhoea26. There is a potential risk of hypernatraemia with this slightly hyperosmolar solution when compared to plasma (285-295 mOsm/L) which may cause osmotically driven increase in stool output in neonates and young infants27. In oedematous malnutrition there is concern of the sodium delivered by ORS. It maybe as high as 9 mEq/Kg/day, which is sufficient to cause heart failure in these children28.
The glucose concentration of 2gms/dl providing 111mmol/L is thought to be sufficient for sodium and water absorption in ORS solution. Higher concentration resulted in osmotic diarrhea. Decreased acceptance due to less sweetness has not been documented. The optimal absorption is supposed to be with glucose to sodium around a molar ratio of 1.4 to 2.0.
Little difference exists in quantity of potassium lost in various types of diarrhea. 20 mEq/L of potassium was thought be adequate. Administration of higher potassium resulted in increased of net absorption but reduced acceptability. Few patients will have difficulties in potassium imbalance once oral replacement has commenced. Malnourished children should be observed for signs of potassium deficiency as the body store is less to start with. Base replacement is in the form of citrate or bicarbonate providing 30 mEq/L which is sufficient to correct mild acidosis from stool loss or because of dehydration.
The standard ORS solution contains 3.5 gms of sodium chloride, 1.5 gms of Potassium chloride, 2.9 gms of Sodium citrate and 20 gms of anhydrous/monohydrous glucose which when dissolved in one liter of water gives the desired concentration of solutes advocated in standard ORS solution. Though for convenience and logistical grounds a single formulation is advocated yet WHO recommended a range for safe and efficacious therapy11.
The total substance concentration should be within the range of 200-311 mmol/L (including that contributed by glucose).
The individual substance concentrations are:
Glucose – Should at least equal that of sodium, but should not exceed 111 mmol/L.
Sodium – Should be within the range of 60-90 mmol/L.
Potassium – Should be within the range of 15-25 mmol/L.
Citrate – Should be within the range of 8-12 mmol/L
Chloride – Should be within the rage of 50-80 mmol/L
During the second half of the standard ORS use numerous studies have been undertaken to develop an improved ORS that would be optimally safe and effective for treating and preventing all types of dehydration from diarrhea and also cause reduced stool output or have other clinical benefits compared to standard ORS. The improvement in quality of ORS was considered from two angles. Firstly, by addition of organic materials like glycine, glutamine, alanine, maltodextrin, rice or wheat powder. As they have alternate apical proteins for carrier mediated coupled transport, it is expected that the water and sodium loss from standard ORS due to high sodium and marginal excess of osmotic load will be reabsorbed resulting in reduction of stool output and improvement in diarrhea.
Secondly, reduction of osmolarity of ORS solution to avoid possible adverse effects of hyperosmolarity on the fluid absorption. This was accomplished by replacing glucose with a complex carbohydrate which gets hydrolysed in the gut to release simple sugars for coupled absorption or by reducing the concentration of glucose and salt in the solution (Low or hypo osmolar ORS).
The formulations containing aminoacids or maltodextrin were not sufficiently effective or practical to replace standard ORS. Rice based ORS is effective in reducing stool output and maintain hydration in comparison to standard ORS and can be utilized if conveniently available. But it is not superior to standard ORS in children with non choleric diarrhea when food is introduced early24.
Reduced osmolarity ORS : The safety and efficacy of reduced Osmolarity ORS in adults with cholera and children with cholera and noncholera acute diarrhea was studied in a multicentric trial in Bangaladesh, Brazil, India, Indonesia, Peru and Vietnam. The study concluded that reduced osmolarity ORS solution (215-245 mOsm/L) with 75 mEq/L or less of sodium and 75-90 mmol/L of glucose is quite safe in comparison to standard ORS solution. These solutions were associated with reduced stool output, reduced vomiting and reduced need for unscheduled intravenous fluids. With sodium <75 mEq/L, the stool output and vomiting are somewhat better than solutions with sodium >75 mEq/L. For children with cholera, the reduced Osmolar solution with 75 mEq/Lof sodium is as effective as standard ORS. This study did not rule out an increased risk of transient asymptomatic hyponatremia. This group of experts recommended the policy of a single solution with 75 mEq/L of sodium, 75 mmol/L of glucose with a total Osmolarity of 245 mOsm/L. Citrate and potassium were of the same concentration as the standard ORS solution. Chloride was reduced to make up the ionic balance to 65 mEq/L11. With reduced osmolarity ORS in children with acute non-cholera diarrhea, a meta-analysis revealed reduced stool output of 20%, reduction of vomiting by about 30% and reduction of unscheduled Intravenous fluid therapy by 39% in comparison to standard ORS solution29.
The choice study group in a multicentric, double blind clinical trial concluded that the need of unscheduled intravenous therapy in Acute Diarrhoea with reduced osmolarity ORS solution is significantly (33%) lower than standard WHO-ORS solution. There is no detectable effect on stool output, illness duration and frequency of hyponatraemia30. The reduction in IV fluid requirement indicates the proportionate decrease in severity.
For the programmatic advantage the Reduced Osmolarity ORS has been recommended by Indian Academy of Pediatrics. However, a plea is made to use a paediatric ORS in non-cholera diarrhea in children with sodium 60 mEq/L, glucose 84 mmol/L and Osmolarity of 224 mOsm/L31.
Nalin DR et al concluded that the reduced Osmolarity ORS is not convincingly superior to standard ORS solution when the latter is used as per recommendations32. The study of S Alam et al does not show significant benefit in use of Hypo Osmolar ORS solution in comparison to WHO ORS. However, the clinical benefits of Hypo Osmolar solution are not denied33.
However the IAP recommended ORS is not available in the market. So also the standard hyper Osmolar ORS has been taken out of the market. At present under the banner of WHO the reduced Osmolar ORS is available.
ORS in special circumstances:
ORS in severe malnutrition
Children with severe malnutrition are deficient in body potassium. In oedematous malnutrition (Kwashiorkor) it is not advisable to give solutions with high sodium content. A formulation with 45 mEq/L of sodium, 40 mEq/L of Potassium with buffered magnesium, zinc and copper with 125 mmol/L of glucose has been advocated by WHO34. In India with infrequent oedematous malnutrition, the reduced osmolarity ORS can be safely used with addition of potassium 3-4 mEq/kg/day and magnesium 0.4-0.6 mEq/kg/day. The fluid should be administered in a slower rate than is recommended for normal children35. Because of the low osmolarity, rice based ORS even with 90 mEq /L of sodium does not produce heart failure and can be used safely36.
ORS in young infants (<2 months of age)
Low Osmolarity ORS with sodium 60 mEq/L, glucose 84 mmol/L and Osmolarity of 224 mOsm/L can be used. If it is not available reduced osmolarity ORS can be used safely without addition of extra plain water31.
Fortification with zinc: Studies reported better outcome with addition of 40 mg of elemental zinc to a litre of standard ORS solution but is less effective to zinc given separately37.
Resistant starch as an adjuvant to ORS: Short chain fatty acids are known to promote water and electrolyte absorption in the colon. Amylase resistant starch which is poorly digested in the small intestine gets fermented by the colonic bacteria to produce short chain fatty acids. This mechanism of fluid and electrolyte absorption being completely different from the carrier mediated coupled absorption, supposed to increase the net fluid and electrolyte retention. However, it is not well tried in non-cholera diarrhea of children35, 38.
The rationale of ORS use: Death from Diarrhoea/Gastroenteritis in children often occurs before hospitalisation or shortly after hospitalization. Comprehensive oral therapy has decreased the deaths in last 3 decades. ORS has reduced the need for intravenous fluids and consequently hospitalization. The cost of ORS is also low and is within the buying capacity of most people in developing countries. However, it cannot be used with intractable vomiting or diarrhea. It is of limited use in severe dehydration with circulatory compromise but can be given after stabilization with intravenous fluids. As “some” dehydration does not produce appreciable electrolyte imbalance there is no need of electrolyte estimation for initiation or follow up therapy with ORS solution.
Method of administration and dose of ORS solution: ORS solution should be given with a cup and spoon. Child with dehydration should be fed 5 to 10 ml per minute. With vomiting dose should be reduced or feeding should be stopped for sometime (10 minutes) and restarted again. With persistent vomiting, Nasogastric feeding should be used. Rehydration itself may reduce emesis7.
The fluid therapy of dehydration consists of three components.
- The rehydration therapy accounts for correction of the existing fluid and electrolyte deficit.
- Replacement of ongoing losses due to continuing diarrhea to prevent recurrence of dehydration.
- The maintenance therapy covers the daily requirements.
A child with diarrhea without dehydration is given ORS solution to counteract the ongoing los in a dose of 10 to 20 ml/kg body weight per each liquid stool. In malnourished children 5-10 ml/kg may be given. However, 10 ml of ORS/kg per each liquid stool is a good working formulation. Breast feeding should be continued and semisolid diets should be started as early as possible. In non-breast fed babies milk mixed with cereals can be used39.
Children with some dehydration should be given 75 ml/kg ORS in first 4 hours. In severely malnourished children the calculated fluid is to be given over 8-10 hours. The maintenance dose o ORS is given over 24 hours. 50 to 100 ml/kg/day along with food and replacement of ongoing loss are adequate till diarrhea subsides. Early initiation of feeding with adequate calorie helps in good weight gain and early recovery.
Conclusion: ORS has significantly brought down the diarrhoeal mortality. Reduced Osmolarity ORS is supposed to bring down the morbidity further. It is a simple effective therapy requiring least scientific skills for administration. Though a single formulation is not best at all situations yet the reduced osmolarity ORS proved meaningful in all ages, all types of diarrhoea irrespective of aetiology, all forms of dehydration where it can be used and in different modes of therapy like deficit correction, maintenance and ongoing loss replacement. Inspite of the drawbacks, Reduced Osmolar WHO-ORS is a significant scientific breakthrough of this century till diarrhoea will cease to be a menace for young children.
Further research:
- The keeping quality of the solution after reconstitution in potable water at different temperatures and humidity is not calculated.
- For 75 mEq/L of sodium the glucose requirement will be 37.5 mmol/L as 2 atoms of Sodium constitutes a molecule. The initial experiment of Sladden and Dawson points to the same.
- Freshly prepared rice pulp or puffed rice pulp is a better candidate to replace glucose and is time honoured and practiced by people. Research in this direction is scanty.
- The existing intraluminal sodium and water is not taken into consideration. Theoretically simple glucose solution will allow absorption of fluid as sodium is already present in Jejunum.
- Addition of resistant starch to ORS as it does not increase the Osmolarity significantly should be brought under the ambit of research.
In ReSoMal the high amount of dextrose should be scanned for its effect on the gut as potassium does not need glucose for absorption. The high Potassium also should to be tested for acceptability.
DECLARATION:
Contribution of Authors: GS- Concept, Writing manuscript ;NM- revising manuscript; NK- Review of literature.
Conflict of Interest: None
Ethical Approval: The study approved by the Institutional Ethics Committee
Funding: Self
References:
1. Diarrhoeal disease – world health organization, may 2 1017, http://wwwowho.int>news-room>fact-sheets>detail>diarrhoeal-disease.
2. Diarrhoeal disease – UNICEF DATA – http://data.unicef.org>topic>child-health>diarrhoeeal-disease
3. National Family health survey 2015- 16 (NFHS-4). rchiips.org/nfhs/factsheet_nfhs_4.sntml.
4. Acute Diarrhoea in Current concepts in the management of Diarrhoea. Eds. Mathur RC, Shah K, Bhave S, Parthasarathy A. PP3-29.
5. Victor CG, Bryce J, Fontaine O, Monasch R. Reducing deaths from Diarrhoea through Oral rehydration therapy. Bull World Health Organ. 2000;78,1246-1255.
6. Water with sugar and salt. Editorial. Lancet 1978; 2:300-301.
7. Acra SA, Ghishan FK. Electrolyte fluxes in the gut and oral rehydration solution. Pediatr Clin North Am 1996; 43:433-449.
8. Harrison HE. The treatment of diarrhea in infancy. Pediatr Clin North Am 1954; 1:335-348.
9. Curran PF. Na+/Cl- and water transport by rat ileum in vitro. J Genet Physiol 1960; 43:1137-48.
10. Slauden GE, Dawson AM. Inter relationships between the absorption of glucose, sodium and water by the normal human jejunum. Clin Sci 1969; 36:119-132.
11. Expert Consultation of Oral Rehydration Salts (ORS) formulation. 2001 Jul 18; UNICEF house, New Work, USA Document wef. WHO/FCH/CAH/ 01. 22.
12. WHO/UNICEF joint statement: clinical management of acute diarrhea. New York: WHO & UNICEF; 2004(WHO/FCH/CAH/04.7). http://www.afro.who.int/cah/documents/intervention/acute_diarrhoea_joint-statement.pdf.
13. Gomez BI, McIntyre MK, Gumey JM et al. Enteral resuscitation with oral rehydration solution to reduce acute kidney injury in burn victims: evidence from a Porcine model. https://doi.org/10.1371/journal.pone.0195615, May 2, 2018.
14. Guideline: Updates on management of severe acute malnutrition in infants and children. In Edited by organization WH Geneva WHO, 2013.
15. Curran PF, Maclntosh JR.A model system for biological water transport nature 1962;193:347-351.
16. Casteel H, Fiedorek SC. Oral Rehydration Therapy. Pediatr Clin North Am 1990; 37:295-311.
17. Davis GR, Santa Ana CA. Morawski SG, et al. Permeability characteristics of human jejunum, ileum, proximal colon and distal colon. Results of potential diference measurements and unidirectional fluxes. Gastro enterology 198283:844-849.
18. Gyton and Hall: Text book of Medical Physiology, 13th edition, 2016.
19. Fordtrans JS, Rector FCJR, Carter NW. The mechanism of sodium absorption in the human small intestine. J Clin Invest. 1968 April; 47(4): 884-900.
20. Pathophysiology of Potassium absorption and secretion by the human intestine. Agarwal R, Afzalpurkar R, Fordtran JS. Gastroenterolgy, 1994 Aug; 107(2) 548-571.
21. Sladden GE. The pathogenesis of cholera and some wider implications Gut, 1973;14:671-674.
22. Molla AM, Rahman M, Sarkar SA, et al. stool electrolyte conent and purging rates in diarrhea caused by rotavirus, enterotoxigenic E. coli and V cholera in children. J Pediatr 1981;98:835-838.
23. Sladen GE and Dawson AM. An evaluation of perfusion techniques in the study of water and electrolyte absorption in mass: the problem of endogenous secretions. Gut 1968, 9, 530-535.
24. WHO. 25 years of ORS – Joint WHO/ICDDR Consultative meeting on ORS formulation – Dhaka, Bangladesh, 10-12 December, 1994. Document ref. WHO/CDR/CDD/95.2 Geneva, World Health Organisation, 1995.
25. Avery ME, Synder JD. Oral therapy for acute diarrhea. The underused simple solution. New Engl J Med. 1990; 323:891-894.
26. Mahalanabis D. Use of an oral glucose electrolyte solution in the treatment of pediatric cholera; a controlled study. Journal of Tropical Pediatrics and Environmental child health 1974; 20:82-87.
27. Bhargaava SK, Sachdev HPS, Das Gupta B, Daral TS, Singh HP, Mohan M. Oral rehydration of neonates and young infants with dehydration diarrhea; Comparison of low and standard sodium content in Oral rehydration solutions. J Pediatr Gastroenterol Nutr 1992; 14:113-115.
28. Golden MHN. Edematous Malnutrition. Med Bull 1998; 54:433-444.
29. Hahn SK, Kim YJ, Gamer P. Reduced Osmolarity Oral rehydration solution for treating dehydration due to diarrhea in children: Systemic review. BMJ 2001; 323:81-85.
30. Choice study group. Multicenter, Randomized, double-blind clinical trial to evaluate the efficacy and safety of a reduced Osmolarity Oral Rehydration salts solution in children with acute watery diarrhea. JAMA 2001; 291:2632-2635.
31. Consensus statement of IAP National Task Force: Status Report on management of Acute Dairrhea. Ind Pediatr 2004; 41:335-348.
32. Nalin DR, Hirschhorn N, Greenough W, Fuchs GJ, Cash RA. Clinical concerns about reduced-Osmolarity Oral rehydration solution JAMA 2004; 291:2628-2631.
33. Alam S, Afzal K, Maheshwari M, Shukla I. Controlled trial of Hypo-Osmolar versus world Health Organization Oral rehydration solution. Ind Pedaitr 2000; 37:952-960.
34. WHO, Department child and adolescent health and development. Management of the child with a serious infection or severe malnutrition. Guidelines for care at the first referral level in developing countries. Document ref. WHO/FCH/CAH/00.1 Geneva, World Health Organization, 2000.
35. Bhan MK. Current concepts in management of acute diarrhea. Ind Peditr 2003; 40:463-476.
36. Ahemad T, Ali M, Ullah MM, et al. Mortality in severely malnourished children with diarrhea and use of Standardized management protocol. Lancet 1999; 353:1919-1922.
37. Bahl R, Bhandari N, Saksena M et al. Efficacy of zinc fortified oral rehydration solution in 6-35 month old children with acute diarrhea. J Pediatr 2002; 141:677-682.
38. Ramakrishna BS, Venkataraman S, Srinivasan P, Dash P, Young GP, Binder HJ. Amylase-resistant starch plus oral rehydration solution for cholera. N Eng J Med 2000; 342:308-313.
39. Singh UK, Praad R, Kumar R, Jaiswal BP. Management of diarrhea inPractice. Indi J Pediatr 2002; 69:687-695.
Issue: July-September 2019 [Volume 8.3]