Genetic study in children with Down syndrome and autism-like behavioural disorders
Original Research
Abstract
Genetic disorders are diseases caused due to an abnormality in an individual’s genetic makeup which affect an individual for life and can rarely be treated. The aim of the study was to understand the phenotypic and behavioural characteristics and constitutive genetic abniormalities in Down syndrome and autistic children through conventional cytogenetic technique. A total of twenty cases were counselled and recruited for the present study. The peripheral blood samples were cultured in complete nutrient medium for chromosome analysis, including karyotyping, and assessment of micronucleus and apoptosis. Gbanding analysis of 25 metaphases in classified form detected the constitutive aberrations and study of 1000 cells enumerated the frequencies of micronucleated and apoptotic cells. Chromosomally all DS children had trisomy 21, and all autistic children had normal karyotypic constitution. The micronuclei count and apoptosis appeared higher in Down syndrome than that of autism, which indicates higher genetic instability and risk of neoplasia. Identification of the genetic etiology and relevant counselling would be helpful for the parents of the genetically impaired children for better understanding of the present and future health, and be better equipped emotionally.
Key words: Genetic disorders; chromosomal abnormalities; micronuclei.
Introduction
Gene-environment interaction increases the disease quantum in normal as well as genetically impaired population with a higher propensity in the latter (Ganguly 2011). Down syndrome (DS) is the most prevalent autosomal disorder with a global incidence of 1 in 700 live births across all communities and backgrounds alike, which was clinically characterized in 1866 and genetically understood in 1959 (http://www.cdc.gov/ncbddd /birthdefects/downsyndrome/data.html). Typical phenotypic characteristics of DS including hypotonia, excessive joint laxity, a flat face with flat nasal bridge, up-slanting eyes, an almond shape to the eyes caused by oblique eye fissures with epicanthic skin folds, low set ears, large space between the big toe and its neighbouring toe (‘sandal gap’), clinodactyly, a higher number of ulnar loop dermatoglyphs, large protruding tongue (due to small oral cavity), hypertelorism, a single transverse palmar fold (Simian crease), short neck, shorter limbs, white spots on the iris known as Brushfield spots, delayed global and cognitive development, mental retardation, etc. distinguishes them from pears of their age (“CDC – Birth Defects, Down Syndrome -NCBDDD”. Cdc.gov. 2013-11-0 6 ) . Systemic complications affecting physiological systems have been reported in 40- 45% DS, including hypo-/hyperthyroidism, congenital heart disease (CHD) with atrio- (ASD) or ventricular (VSD) septal defects (~50%), failure to thrive due to nutritional deficiencies, especially in children with severe CHD, obesity at adolescence and early adulthood, pre-mature senility, hearing and speech impairment, cataract, Hirschsprung disease, Alzheimer disease, skeletal problems, obstructive sleep apnea, anemia and so on (Ganguly and Kadam, 2017).Cytogenetic techniques which include karyotyping, fluorescence in situ hybridization (FISH) and comparative genomic hybridization (CGH) are essential to observe the chromosomal abnormalities in people with congenital disorders (ISCN 2016). Karyotyping is the process of pairing and ordering all the chromosomes of an organism. Down syndrome is caused by an extra copy of the chromosome 21 (trisomy 21 or HSA21) in all or fraction (mosaicism) of the cells.
Autism is a complex developmental disability. Clinical expressions typically appear during early childhood and affect a person’s ability to communicate, and interact with others. Autism is a “spectrum condition” (also known as autism spectrum disorder or ASD) that affects individuals differently and to varying degrees. Some of the behaviours associated with autism include delayed learning of language, difficulty in making eye contact or holding a conversation, difficulty with executive functioning; which relates to reasoning and planning; narrow, intense interests; poor motor skills and sensory sensitivities and so on (Sigman et al. 1999). Its incidence rate is four times more in boys than girls (1 in 42 boys and 1 in 189 girls). The qualitative and quantitative parameters, including weight, behavioural characteristics, vision, effect of parental age, consanguinity, physical abnormalities present and health problems the special children suffer from and genetic instability (micronuclei), apoptotic index, chromosomal count, advanced maternal age, consanguinity and proband’s weight at birth respectively were studied in this report with a view to comparing the parameters between the two study groups.
Material and methods
The study was carried out with collaborating support from two institutions such as Spandan
Holistic Mother and Child Care Hospital, Govandi, Mumbai and Swami Brahmanand Pratishthan, CBD Belapur, Navi Mumbai. Pre- test counselling was conducted with the parents of the children having DS and ASD. The parents were thoroughly explained about the process of the test on a one-on-one session conducted for each parent to note down their family & medical histories, and benefit and possible adverse effects from participation in the study. The children of the consented parents only were recruited in the study. A total of 20 parents had agreed to participate in the present study. However, peripheral blood samples could be collected from 15 children who had the history of mental retardation and delayed milestones (DS: 11; ASD: 4). The parents were given a karyotype report of all the participants and explained with clarification of their doubts individually. Approval was obtained from the Institutional Ethic Committee (IEC).
Approximately, 2-3 ml of peripheral venous blood was collected from each individual in sterile sodium heparin vacutainers. The samples were collected in the premises of the two institutions and transported to the genetic testing lab within 2-3 hours for further processing. The blood samples (0.5ml) were cultured in RPMI 1640 nutrient (4ml) medium supplemented with fetal bovine serum (FBS) (20%) and phytohaemagglutunin (PHA) (all from Gibco, USA) and maintained at 37°C for 72 hours incubation (Ghosh 1988 ). Harvesting of cells carried out following colchicines-hypotonicfixative schedule. Colchicine was added 1 hour prior to the completion of 72 hours followed by incubation for 1h. The samples were centrifuged at 1000
RPM for every change and washing of cells from debris till a clear supernatant was obtained.
The cell suspension was dropped onto chilled glass slides from a height of ~12 inch using a Pasteur pipette to cover the entire surface. The slides were air dried by putting them on a hot plate (~50-60oC), and trypsinised for GTG-banding followed by staining in Giemsa. Geneticanalysis, including karyotyping (25 metaphases) following ISCN classification and frequency of micronucleated and apoptotic cells (1000 nucleated cells).
Results
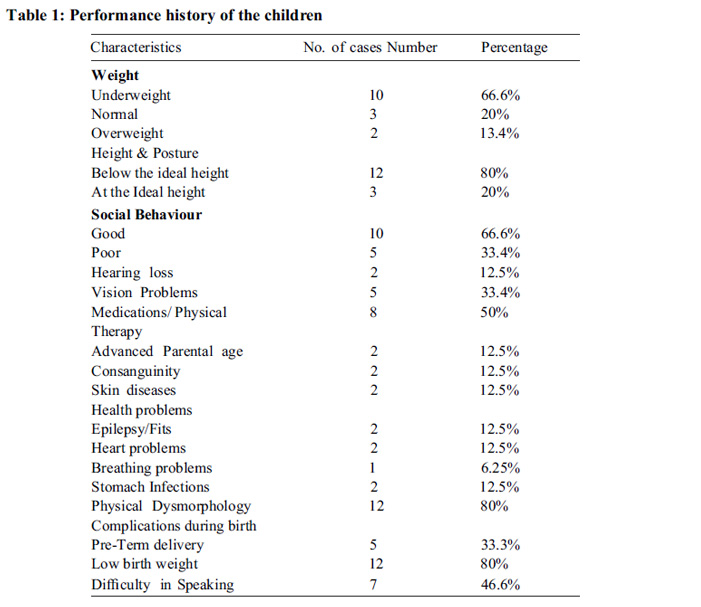
Phenotypic and clinical characteristics are presented in table 1. Qualitative parameters revealed 72% of the DS cases to be underweight and 50% of the ASD cases were overweight; 81% of DS cases showed good social behaviour and cases with ASD had communication problems.
Conductive hearing loss was observed in 12.5% of DS cases while ASD showed no problems with hearing. Vision issues such as peripheral vision and occasional black-outs were observed in 18.75% of ASD cases. Majority of the DS cases wore spectacles to address their vision problems. Skin diseases were seen in 12.5% of DS cases. Health Problems including stomach infections, epilepsy (seizure), and shortness of breath and congenital heart disorders (ASD/VSD) were observed equally affecting the cases of DS and ASD. For achieving muscle tone, 50% of the total cases were undergoing some form of medication and occupational therapy. DS cases having ASD, didn’t talk at all (50%), while 45.5% DS cases had difficulty in speaking in general.
Consanguinity was observed in 12.5% of the total cases. Premature delivery with low birth weight was seen in 45% of the total cases. Of all, 13.3% of cases had mothers of advanced maternal age and 46.6% cases had fathers of advanced paternal age at birth (Fig.1).
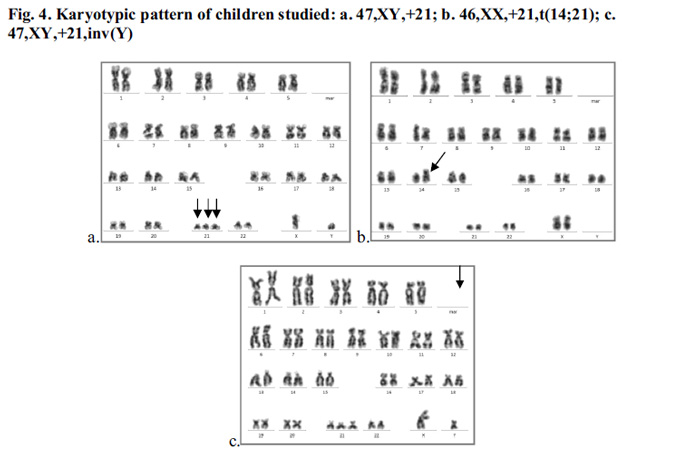
Among the 11 DS cases, 18.18% of adolescent cases (aged 24.83±2.23 years) had micronuclei count between 35 to 40 and 81.81% of children (aged 6±4.24 years) and (14±2 years) had micronuclei count between 0 to 11 and 27 respectively (Fig.2a). For ASD, 50% children of age6.5±0.71 years had micronuclei between 10 to 15 and the remaining of 13.5±4.95 years age having micronuclei between 35 to 40 (Fig.2b). Apoptotic incidence were appeared as 27.27% DS cases having apoptotic cells >60, 27.27% cases having between 30 to 60 and 45.45% of the cases had below 30 (Fig.3a). Among ASD, 25% had apoptotic cells >150 and 75% of cases had apoptotic count to 100 (Fig3b). The chromosomal count of 81.81% of DS cases was 47 (47, XX,+21 or 47,XY,+21) (Fig.4a) and the remaining 18.18% cases had balanced Robertsonian translocation (46,XX,rob(14;21)) (Fig. 4b). In addition, one DS child had a pericentric inversion in Y chromosome (inv(Y)) (Fig.4c). All ASD cases showed apparently normal karyotype with 46,XX or 46,XY chromosomal pattern.
Discussion
Children born with chromosomal abnormalities are subjected to a spectrum of delayed mental and developmental milestones, and phenotypic and clinical manifestations (Ganguly and Kadam 2017; Weijerman and Winter 2010). Autistic spectrum disorder (ASD) being a multifactorial disorder often causes behavioural expression mostly by lacking eye contact and social response (Sigman et al. 1999; Simmons et al. 2009). However, on time special care and training help them developing social and vocational skill. In the present study, 81% of children appeared obedient and co-operative. Facial features including upslanting and palpebral fissures, epicanthic folds and small white Brushfield spots on the iris of DS children contribute to poor vision. Autistic children mostly have normal focal vision but sometimes peripheral vision cases are seen in which they look at people from the corner of their eyes and not make direct eye contact while conversing because they can’t process auditory and sensory information together. Although ASDchildren didn’t have typical phenotype, broad forehead, wide set eyes and expressionless face was noted in 75% of all cases in the present study. Many of the DS pregnancies die in infancy and early adulthood, or at uterine age. Although the trisomy Hsa21, is the same in most DS individuals, the penetrance of the resulting pathologies is varied as most DS have memory and learning difficulties, craniofacial alterations and muscle hypotonia, but only some have congenital heart defects, leukemia or gut abnormalities (Nižetić and Groet 2012). Furthermore, the severity of the defects is variable with varying cognitive impairment. Variability in clinical expression could be contributed by the site of meiotic recombination of bivalents, chromosomal polymorphisms, epigenetic factors and gene dosage (Ganguly and Kadam 2017).
Age of the parents at birth, especially maternal age, is the main causal factor for birth of Down syndrome. Women over age of 35 are at risk of having children affected with genetic disorders, mostly DS (Ganguly and Kadam 2017). However, younger mothers can also have meiotic nonr disjunction and reproduce DS children. In the present study, some of the DS children were born to younger mothers. Increased paternal age and age gap between the two parents were also reported to cause genetic defects in children (Shelton et al. 2010). Consanguinity and genetic anomaly in parents are known to cause abnormalities in children. Consanguineous marriage doubles the risk of transmission of genetic defects. The DS child with t(14;21) in the present study was born to consanguineous parents; however, parental transmission of the translocation could not be confirmed due to lack of their co-operation. Similarly, paternal inheritance of inv(Y) in the DS child remained unknown.
Trisomy 21 is the known genetic abnormality in DS population resulting in aneuploidy with 47 chromosomes. However, 2-5% DS children carry hereditary t(21;21) or de novo variantRobertsonian translocation with no numerical changes (Ganguly and Kadam 2017). ASD could be a poly-gene disorder caused by multiple factors. However, irrespective of the etiology, children born with genetic impairment are at higher risk of acquiring mutations and developing cancer. DS children suffer from leukemia (Ganguly et al. 2017a; Hasle et al. 2000). Increased frequency of spontaneous incidence of micronuclei in the present study might indicate their risk in future health. Spontaneous apoptosis indicates natural death of the lymphocytic cells.
In view of the above findings, the present report highlights the necessity of chromosome/ genetic analysis of children born with congenital malformations and/or having delayed milestones and behavioural disorders for understanding the genetic etiology of clinical expression. Study of micronucleus formation indicates accumulation of abnormalities, which further indicates the necessity of health surveillance. However, co-operation of parents and/or blood-linked relatives would be essention for such investigation (Ganguly et al. 2017b). The study needs to be carried out on larger sample size with a positive attitude of the parents for extended family study as and when necessary.
Acknowledgements
The authors gratefully acknowledge the support of the Mahatma Gandhi Mission Trust for facilitating the investigation. The authors also thank the parents of the participating children for
consenting for the study.
References
1. Ganguly BB. Chromosomal Abnormalities in Man. In: Perspectives in Cytology and Genetics (Giri AK ed). 2011; 15: 189-198.
2. Ganguly BB, Kadam NN. Down syndrome: from the age of characterization to the era of curative approach. Nucleus 2017; 60:19 7 – 208. DOI 10.1007/s13237-016-0187-y
3. Ganguly BB, Kadam NN, Mandal PK. Complexity of chromosomal rearrangements in Down syndrome leukemia. J Can Res Ther 2017a;13:381-3.
4 . Ganguly BB, Daruwalla D, Kadam NN. Attitude of parents to carrier screening of genetically transmitted diseases in India. MGM J Med Sci 2017b; 4(4): 187-190.
5. Ghosh BB. (1988). Alterations in Structure and Behaviour of Chromosomes and Certain
6. Cellular Components Induced by Heavy Metal. Ph.D. Thesis, University of Calcutta, Kolkata, India.
7 . Hasle H, Clemmensen IH, Mikkelsen M. Risks of leukaemia and solid tumours in individuals with Down’s syndrome. Lancet 2000; 355(9199):165-9. http://www.cdc.gov /ncb ddd / birthdefects/downsyndrome /data.html.
8. “CDC—Birth Defects, Down Syndrome- NCBDDD”. Cdc.gov. 2013-11-06.
9. ISCN. An International System for Human Cytogenomic Nomenclature. Eds: McGowan
10. Jordan J, Simons A, Schmid M. S. Karger AG, Basel (Switzerland), 2016.
11. Nižetić D, Groet J. Tumorigenesis in Down’s syndrome: big lessons from a small chromosome. Nat Rev Cancer 2012; 12(10):721-32. doi: 10.1038/nrc3355. Epub 2012 Sep 21.
12. Sigman M, Ruskin E, Arbelle S, Corona R, Dissanayake C, Espinosa M, Robinson B.
13. Continuity and Change in the Social Competence of Children with Autism, Down Syndrome, and Developmental Delays. Monographs of the Society for Research in Child Development 1999; 64(1), I-139. Retrieved from http://www.jstor.org/ stable/3181510
14. Simmons DR, Robertson AE, McKay LS, Toal E, McAleer P, Pollick FE. Vision in autism spectrum disorders. Vision Research 2009; 49(22),2705–2739. https://doi.org/ 10.1016/ j.visres. 2009.08.005
15. Shelton JF, Tancredi DJ, Hertz-Picciotto I. Independent and dependent contributions of advanced maternal and paternal ages to autism risk. Autism Research 2010; 3(1): 30-9. Doi:https://doi.org/10.1002/aur.116
16. Weijerman, ME; de Winter, JP. “Clinical practice. The care of children with Down syndrome.” European journal of pediatrics 2010; 169 (12): 1445–52. doi: 10.1007/ s00431-010- 1253-0. PMID 20632187.
Issue: April-June 2018 [Volume 7.2]